Abstract
Understanding complex cell–cell interactions and physiological microenvironments is critical for the development of new therapies for treating human diseases. Current animal models fail to accurately predict success of therapeutic compounds and clinical treatments. Advances in biomaterials, engineering, and additive manufacturing have led to the development of printed tissues, lab-on-chip devices, and, more recently, organ-on-chip systems. These technologies have promising applications for the fabrication of more physiologically representative human tissues and can be used for high-throughput testing of human cells and organoids. These organ-on-chip systems can be fabricated with integrated fluidics to allow for the precise control and manipulation of cellular microenvironments with multiple cell types. Further control over these cellular environments can be achieved with bioprinting, allowing for three-dimensional (3D) printing of multiple materials and cell types to provide precisely controlled structures manufactured in a one-step process. As cell behavior is highly dependent on the physical and chemical properties of the environment, the behavior of cells in two-dimensional and 3D culture systems varies drastically. Providing devices that can support long-term cell culture and controlled stimulation of 3D culture systems will have a profound impact on the study of physiological processes and disease, as well as the development of new therapies. This review highlights recent advances in organ-on-chip systems and 3D bioprinting techniques for the development of in vitro physiological models.
Export citation and abstract BibTeX RIS
Introduction
Organ-on-chip systems are versatile tools that can be used to recreate complex tissues in vitro to study human physiology and guide drug development. The key to creating representative ex vivo models is to incorporate relevant cell populations that mimic physiological structures in emulated tissue microenvironments [1]. Commonly used fabrication techniques (such as lithography and gel confinement) for the development of organ-on-chip systems have a limited capacity to create tissue structures with complex three-dimensional (3D) organization [1, 2]. To develop platforms housing organized cell and tissue constructs, 3D bioprinting offers the ability to construct tissues layer-by-layer using tailored scaffold materials with high-precision cell deposition [2]. The synergistic application of 3D bioprinting to construct tissues in organ-on-chip bioreactors has the potential to revolutionize in vitro organoid models through the inclusion of complex physiological structures in controlled extracellular environments.
In this review, the current progress of organ-on-chip systems utilizing fluidic and/or 3D bioprinting technologies is discussed. Each section will discuss the relevant physiological structures and interfaces that are necessary to mimic specific organ function and disease behavior outside of the body and describe the fluidic and bioprinting methods used to achieve effective emulation. Devices that incorporate both 3D bioprinting and fluidics will be highlighted and potential for further integration of the techniques will be summarized.
Biological fluidic systems
Fluidic devices, particularly microfluidics systems, have an extensive history of being used to miniaturize technology to reduce costs and manage smaller samples. Their use began with the genomics boom of the 1980s, continued with the biodefense programs of the 1990s, and are at the forefront of medicine today [3]. Microfluidics is the field of study regarding systems that handle small volumes (10−9 to 10−18 l) of fluid through channel dimensions on the order of one to hundreds of microns [3]. The primary benefit of these systems is the ability to finely manipulate and characterize small volumes of fluids; these devices maximize control over the microenvironment and minimize the use (and waste) of costly reagents or small biological samples. This technology has revolutionized the biomedical field as it facilitates the inexpensive production and use of devices used to study micro-scale interactions, particularly those of biological samples. Successful applications of microfluidics have made a large impact across science and medicine. For example, the integration of microfluidic technology with polymerase chain reaction (PCR) has facilitated faster, cheaper, and more precise analysis of nucleic acids [4]. Other systems have been developed to easily observe minute quantities of fluids to better understand and characterize chemical and biological reactions [5]. Microfluidic devices also have translational potential as demonstrated by the development of inexpensive point-of-care diagnostic devices [6] with the ability to identify infectious diseases, such as HIV/AIDS [7] and Lyme disease [8], as well as medical conditions, such as male infertility [9]. These technological advances have influenced the generation of many in vitro biological models through their minimal reagent consumption to achieve scalable results.
Organ-on-chip platforms are fluidic devices containing organized biological structures that emulate the physiological function, behavior, and response of their analogous organs in the human body [10, 11]. While there is extensive diversity amongst organ-on-chip platforms, each of them shares the same goal: to recreate the structure and physiological behavior of human organs outside of the body. These systems have been developed in university labs, national labs, federally funded research centers, and biotechnology companies across the world and hold great promise to improve the understanding of physiological systems, as well as facilitate the development of the next generation of medical treatments [12, 13]. Organ-on-chip systems have been used to study both normal physiological behavior [13, 14] and disease mechanisms [15, 16] in vitro over extended periods of time ranging from weeks to months. The introduction of organ-on-chip platforms in the drug development pipeline can significantly reduce the cost associated with animal testing and prevent ineffective and/or toxic compounds from proceeding into clinical trials [17]. Additional applications include high-throughput drug screening and personalized medical practices for diagnosis and treatment optimization [18].
3D Bioprinting
3D bioprinting is an advanced fabrication technology that is used for creating tissues of one or more cell types that can mimic the 3D geometry and structure of native tissue [19, 20]. While biomedical research has historically used two-dimensional (2D) cell culture for many models, 3D culture methods are gaining popularity as they have been shown to produce more relevant physiological responses in many situations; this topic has been covered in depth in another review article [21]. Bioprinting is a relatively new field, with the early '3D bioprinting' journal articles being published in 2006 [22–24]. Although the field of 3D bioprinting is young, the potential to print human organs stems from the invention of stereolithography by Charles Hull in 1983 [25, 26]. The invention of stereolithography gave rise to the field of additive manufacturing and led to the manufacturing of the 3D printer in 1986 [25]. Additive manufacturing was originally used with materials such as resin and acrylics. The use of additive manufacturing in machine shops and other fields eventually inspired medical researchers to consider the use of 3D printers to construct human organs [25]. Almost 15 years later, in 1999, Atala et al created a human bladder scaffold by 3D printing a polyglycolide (PGA) scaffold [27]. Bladder biopsies and muscle cells were harvested and expanded. Within five weeks of isolation, urothelial and muscle cells were seeded onto the printed bladder. Their group's work recreating a hollow organ represents a new frontier in tissue engineering and was widely recognized for its innovative application of existing technologies [27]. The strategy of 3D printing a scaffold and then seeding cells onto it led the way for a new field, called 3D bioprinting. Bioprinting differs from 3D printing in that it prints cell-laden bioinks, versus 3D printing, in which the inks do not contain cells [19].
The strength of 3D bioprinting lies in the ability to precisely control the placement of printed cells and biomaterials [27]. Applications of 3D bioprinting can be divided into several categories: organ fabrication for the purpose of performing an in vivo function (such as blood filtration), tissue fabrication for use as in vitro models for pharmaceutical drug testing, and physiological research [28]. 3D bioprinting of tissues for implantation and drug development can offer many advantages, including decreased costs, automation, and reproducibility [28]. In addition, 3D bioprinting can address one of the limiting factors of other tissue engineering strategies, size [29]. Large tissues are difficult to make with other in vitro techniques due to mass transfer limitations [29]. Introduction of vascularization can overcome these constraints and allow for the creation of larger tissues. 3D bioprinting can drastically improve vascularization in tissue engineering, a challenge which has yet to be surmounted by other techniques [30]. Addressing this limitation will be a large step in the clinical translation of bioprinting-generated tissue and organ constructs [30].
Vasculature
Vasculature is the highway for nutrients and waste in the body. Humans and other vertebrates have two kinds of vasculature that share a developmental origin but offer different functions. Blood vessels in the circulatory system export oxygen and small nutrients to the body and return chemical wastes, and lymph vessels drain interstitial fluid to the circulatory system while providing a battleground for the immune system. Both provide systemic molecular transport, which is key for drug transport and maintaining homeostasis. Vasculature-on-chip provides a platform for cross-talk between multiple cell and tissue types to replicate pathological function and physiological conditions [31, 32]. Consequently, vasculature is considered a minimal requirement for any engineered tissue [33, 34]. Vasculature-promoting cells such as human umbilical vein endothelial cells (HUVECs), embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are included in bioinks, or in matrices that act in conjunction with bioinks, to produce these vasculature-on-chips and supplement other organ-on-chip systems [35]. Recent reviews have discussed in detail the body of work in bioprinted vasculature-on-chip systems [30, 36, 37]. Here, we highlight work in vasculature that has implemented microfluidics or bioprinting and the integration of the two for biomimetics in angiogenesis, vascular disease, lymphatic tissue, and implementation in other organ-on-chip systems.
Vasculature-on-chip technology was first used for by observing and understanding healthy vessel physiology, including thrombosis and angiogenesis, in microfluidic systems [38, 39]. Better understanding of healthy physiology allows for improved comparison to diseased states, such as the pathophysiology of congenital diseases, cancer, and cardiovascular diseases [38]. A leading cause of cardiovascular disease is atherogenesis. In a recent study, mechanical application of shear stress through a pneumatic air-ballooned membrane coupled with a chemical stimulus, exposure to tumor necrosis factor-alpha (TNF-α), caused leukocyte adhesion, a key first step in developing vessel-clogging plaque. Increasing the vessel model's restriction from 50% to 80% caused overexpression of intercellular adhesion molecule-1 (ICAM-1) [40]. Mechanical manipulation aided the group in creating the tunable 3D vessel model for noncommunicable disease. Another example of such a device replicated hereditary hemorrhagic telangiectasia (HHT), a cardiovascular syndrome that manifests in arteriovenous malformations (AVMs) without capillaries which are subject to frequent bursting [39]. By inhibiting growth factors the growing vasculature disorganized, causing shrunken vessels, typical of malformations of HHT. This demonstrated the physiology of the disease by modifying the cellular signals local to the tissue, which disrupted angiogenesis. Further, the monitoring of angiogenic factor release and heterogenous vascularization, or angiogenic invasion of a tissue, in tumor models has been made possible by developments in implementing vasculature to tumors-on-chips [41]. Several of these are discussed in a later section of this paper. The purpose of emulating vascularization in these systems is to show the growth of full blood vessels in a cancerous tissue. This contrasts with endothelialized organs-on-chips, in which a layer of endothelium is seeded on an existing tube.
Endothelialized structures aid in analyzing the interactions between flow, blood cells, and the vessels themselves. Tsvirkun et al analyzed in detail the presence of a glycocalyx layer covering the endothelium of a blood-vessel-on-a-chip under physiological flow, allowing a fluid shear stress of 0.2 Pa [42]. The surface layer from the seeded HUVECs was around 600 nm, which reflected conditions in vivo, whereas 2D cell culture developed a layer of around 10 nm. The development of an extracellular matrix (ECM) relevant to the physiological structure in vivo allowed for tunable blood flow analysis, of which the group called for further investigation. In recent years, an article was published containing simple, easy-to-follow instructions to construct endothelialized 3D in vitro vessel models with customizable features including stenosis (vessel narrowing), aneurysms (vessel ballooning), and bifurcation (split vessel) [43]. The materials for these do-it-yourself (DIY) vessels-on-chips are common and relatively inexpensive, although the technology is not ready for mass production [44].
More recently, another step towards physiological accuracy in vasculature-on-chip systems was taken when Shöneberg et al bioprinted a multi-layer blood vessel with 83% smooth muscle cell (SMC) viability, showing no statistical difference from cells pipetted onto the same hydrogels. While others have reported seeding cells in microfluidic chips to fabricate multilayered vessels, this group utilized a custom bioprinter [45, 46]. The bioprinter was custom-built with three printheads, with one containing HUVECs, a second containing SMCs, and a third containing thrombin for crosslinking. The outer, middle, and inner layer, composed of fibroblasts with ECM, SMCs, and HUVECs, respectively, resembled those found in vivo, creating a vessel about 1 mm in diameter with walls of 0.43 mm thickness. The inner layer of HUVECs expressed cadherin and cluster of differentiation 31 (CD31) molecules, which are essential for intercellular communication and tissue formation. Adding multiple layers of tissue greatly improves biomimicry of the controllable fluidic systems [46]. This system did not rely completely on 3D bioprinting, however, as the outermost layer of fibroblasts was cast onto the muscle cell layer. Improvements can still be made on the current technology to completely 3D-print vasculature from cultured cells.
A central limitation of in vitro tissue engineering strategies is the lack of an adequate blood vessel supply [47]. In vivo, a network of blood vessels supplies tissues with nutrients and oxygen [47]. Organ-on-chip platforms are restricted in size and complexity without vasculature-on-chip since diffusivity of nanoscale molecules through the ECM drops as though in a viscous fluid (1.34 times viscosity of water) [48]. During embryonic development, animals overcome the limitations of diffusive gas and small-molecule transfer by developing vascular networks [49]. Similarly, transferring nutrients and waste from small (1–2 nm) molecules to large proteins (10 nm) requires bulk transfer of fluids throughout an organ-on-chip. Dense vasculature systems are required to sustain thick (>3 mm) organ-on-chip systems for longer periods of time [50]. Large-scale fluidic systems only remain viable if a robust vascular network continues to survive engineered tissues [51]. The 3D bioprinted vasculature-on-chips have thus far produced mostly thin, short-lived vascularized systems. Recently, Kolesky et al developed a method to create a thick (>1 cm) and long-lasting (>6 weeks) bioprinted vascular system using a sacrificial bioink, composed of Pluronic and thrombin (figure 1) [52]. This fugitive bioink was printed in a crosshatched pattern to a thickness of 1 cm. Next, a surrounding matrix, containing HUVECs, hMSCs, and human neonatal dermal fibroblasts (HNDF), was 3D bioprinted around the crosshatched structure. Removal of the fugitive ink resulted in the formation of a connected network of vessels that supported endothelialization and retained cell viability up to 95%. After creating this structure, Kolesky et al found that adding osteogenic media to hMSC-printed tissue formed symmetric, bone-like tissue after 30 days. Previously, agarose also proved to be a viable sacrificial ink that could provide vessels in a matrix without the reported cytotoxicity associated with Pluronic [53].
Figure 1. Bioprinting vasculature for organ-on-chip applications. (A) Illustrated fabrication protocol for the production of hollow channels in a hydrogel. (i) An extrusion-based bioprinter prints agarose fibers. (ii) A hydrogel precursor is poured over the 3D bioprinted agarose and photocrosslinked. (iii) Agarose fibers are removed. (iv) Fully perfusable microchannels are formed. Reproduced from [53] with permission from The Royal Society of Chemistry. (B) Schematic of the vasculature-on-chip tissue fabrication process. (i) Fugitive Pluronic and thrombin and gelatin-fibrinogen cell-laden inks are printed into the fluidic chamber. (ii) Gelatin-fibrinogen-thrombin ECM hydrogel is cast over the printed inks. Thrombin induces rapid polymerization of molten ECM into a gel. Transgluatminase slowly cross-links the gelatin and fibrin. (iii) Upon cooling, the fugitive ink liquefies and is evacuated, leaving behind a vascular network, which is (iv) endothelialized and perfused via an external pump. Reproduced from [52] with permission from PNAS.
Download figure:
Standard image High-resolution imageWhile progress is steady in 3D bioprinting circulatory vasculature for 3D organ-on-chip systems, little work has been done to advance 3D bioprinting in the closely-related lymphatic vasculature [19, 54]. Casting and cell-seeding have been implemented in the past to fabricate human lymph-nodes-on-chips to acquire real-time data for immune system response and antigen uptake after treating tumor tissue with anti-cancer drugs [55]. These microfluidic chips have been applied to study the effects of the anti-cancer drugs rhomidespin and IFN-α2b on dendritic cell antigen-uptake and subsequent phagocytosis of apoptotic tumor cells. The research took a closer look at the human immune response post-cancer treatment in real-time, establishing a human cell-based alternative to in vivo animal testing. As the lymphatic system plays an important role in immune response, wound healing, and metastasis, future research could be guided towards promoting the lymphatic system to the list of 3D bioprinted organ systems already established [19].
Developments in 3D bioprinting offer improvements in the structure and function of vasculature-on-chip models. Bioprinting improves upon existing casting and seeding methods, resulting in increased resolution, customizability, and automation [19, 44]. Bioprinters increase the accuracy of cell placement by creating physiological structures based on templates of native patient vasculature captured via magnetic resonance imaging (MRI). The 3D-printed vasculature-on-chip systems can then be mechanically manipulated to elicit physiological response [56]. Mechanical strength of 3D bioprinted vessels-on chips is greater than cell-seeded structures, as shown in work by Gao et al [57]. The authors suggested that the 3D bioprinted vessel could be implemented for training in surgery and in studying vessel physiology in vitro. Cui et al demonstrated that a 3D-printed vascular network control improved osteogenesis and regeneration relative to static culture [58]. As vasculature is vital for tissue growth, these findings encourage integration of 3D bioprinted vessels with organs-on-chips. Progress in organ-on-chip and bioprinted models of vasculature is summarized in table 1.
Table 1. Summary of progress related to vasculature.
| Reference | Description | Major Advance |
|---|---|---|
| Vasculature-on-chip (without bioprinting) | ||
| van der Meer et al [39] | Created a model for hereditary hemorrhagic telangiectasia (HHT) to relate growth factor inhibition to physiology | Demonstrated drug effects on vascularization in a microfluidic vessel |
| Tsvirkun et al [42] | Simulated blood flow through an endothelialized fluidic vessel | Observed endothelial surface layer in cell-seeded vessel-on-chip |
| Mannino et al [43] | Created endothelialized models of stenosis, bifurcation, aneurysm | Established a simple protocol for creating variations of vasculature-on-a-chip |
| Hasan et al [59] | Made a multilayered microfluidic vessel in photocrosslinkable hydrogel | Demonstrated permeability and tunability of thickness in a multilayer vessel |
| Zheng et al [38] | Used lithography to form endothelialized microfluidic vessels within a collagen matrix | Elucidated the angiogenic activities of the endothelia |
| Venugopal et al [40] | Monocytes were perfused over inflamed human umbilical endothelial cell monolayer at different channel constrictions | Facilitated study of hemodynamics and leukocyte-endothelial interactions |
| Sakaguchi et al [51] | Triple-layer cardiac cell sheets co-incubated with endothelial cells that migrate | Vascularized tissue constructs that overcame tissue thickness limitations |
| Vasculature-on-chip (with bioprinting) | ||
| Schöneberg et al [46] | Used drop-on-demand bioprinting to print human umbilical vein endothelial cells (HUVECs) and dermal fibroblasts in fibrin and collagen in native blood vessel configurations, which were then cultivated in fluidic bioreactors | Achieved perfusable vessel models with a biofunctional multilayer wall composition |
| Lee et al [50] | Printed HUVECs in a collagen scaffold perfused with physiological shear | Model for investigating mechanisms vascular remodeling under flow conditions |
| Kolesky et al [52] | HUVECs, neonatal dermal fibroblasts, and bone-marrow derived mesenchymal stem cells printed a gelatin fibrinogen ink | Vascularized, thick (>1 cm) tissues that can be perfused on a chip |
| Bertassoni et al [53] | Micromolding strategy using bioprinted template fibers fabricated microchannel networks in multiple hydrogel materials | Effective technique for vascularization of hydrogel constructs |
| Gao et al [60] | 3D vascular structures (fibroblasts, smooth muscle, and endothelial cells) with multi-level fluidic channels fabricated by extrusion-based bioprinting | Structural similarity, sufficient mechanical strength and biocompatibility to be used a new strategy for printing functional vessels |
Skin
Skin is a large, intricate organ with many important physiological functions [61–63]. The skin serves as a barrier, protecting the body from pathogens, toxic chemicals, mechanical disturbances, and ultraviolet radiation [64]. In addition to its protective functions, the skin has passive functions, i.e. prevention of dehydration, maintenance of oxygen, nitrogen, and hydrogen gas concentrations, and thermoregulation [61, 62]. The skin's accessibility and large surface area have led to its consideration as a route for drug delivery [61]. Skin models are of interest for both risk assessment of pharmaceutical compounds in healthy tissue and for modeling diseased tissue states, i.e. fibrosis and tumor models [62]. The human skin's physiological functions and potential as a method for drug delivery have led to an urgent need to develop improved skin models [61].
Recently, skin-on-chip models have emerged, largely due to the ability of integrated fluidics to recapitulate the mechanical forces and biochemical gradients in the human skin's 3D microenvironment [61]. While skin-on-chip system models mimic the native microenvironment with higher fidelity, they are also lower in cost and able to be maintained for longer time than other pre-clinical models. In 2014, Abaci et al developed a microfluidic skin-on-chip system using human skin equivalents (HSE), which are engineered substitutes composed of primary human skin cells and ECM components [65, 66]. The platform was comprised of two polydimethylsiloxane (PDMS) layers, which were separated by a porous polycarbonate membrane. The upper PDMS layer contained a circular compartment to house the HSEs, while the lower layer had five parallel microchannels. The microchannels were constructed with dimensions determined by previously reported blood residence times in human skin tissues, recapitulating physiological molecular transport and allowing for this HSE-on-chip to be integrated with other organ-on-chips in the future. The design of this HSE-on-chip allowed for pump-free, long-term maintenance of HSEs. Additionally, this model established an air-epidermal interface, a critical component needed for the maturation and differentiation of HSEs. Another advantage of this platform is the 36-fold reduction in the quantity of both cells and media required for maintenance, compared to that needed for more traditional platforms, i.e. Transwell assays. One of the skin's primary functions is to serve as a mechanical, water, chemical, thermal, immunological and microorganism barrier [67]. This platform was validated to maintain skin barrier function for three weeks, via a transdermal transport model. The barrier function of these constructs was evaluated by adding a solution of oligonucleotides tagged with fluorescein amidite on the surface of the skin constructs. Media was collected and the concentration of dye was calculated. The measured concentration in the media showed that the permeability of oligonucleotides through the HSE was not significantly different in week 1, 2, or 3, validating that the barrier function was maintained for three weeks. In addition, this platform was used as a drug testing model for doxorubicin treatment, observing cell structure.
This HSE-on-chip showed promise as a more accurate and lower cost alternative to other in vitro platforms for the testing of drug candidates on skin tissue. Another skin-on-chip model from Wufuer et al co-cultured epidermal, dermal and endothelial cells (ECs) in three separate layers, each separated by a porous membrane to allow for interlayer communication [68]. Immortalized human keratinocytes (HaCaTs), fibroblasts, and HUVECs were cultured in the three layers, respectively, mimicking the epithelial and endothelial barriers. TNF-α was applied to the fibroblasts, or dermal layers, to induce both skin inflammation and edema. Expression levels of pro-inflammatory cytokines were measured to demonstrate the system's ability to recreate in vivo inflammatory response. In addition, the system was then treated with dexamethasone, a popular drug used for treating inflammation. Dexamethasone reduced the TNF-α induced inflammation and edema, validating the system's utility as a model for both the study of disease and for drug testing.
Traditional culture systems are limited by their weak recapitulation of the skin's barrier function [69]. One probable cause of this weakness is the lack of mechanical forces and dynamic flow in traditional culture systems. In 2017, Sriram et al combined a dermal matrix, composed of fibrin, with organ-on-chip technology, creating a full-thickness human skin-on-chip [69]. Their system was comprised of a fluidic bioreactor, with dermal matrix cast inside of the culture chamber, seeded with keratinocytes. The basal side of the dermal matrix was shielded from shear stresses by a support membrane. This design resulted in shear stresses acting only on the external layer of the epidermis and the cells inside. Interstitial flow enhanced the transport of nutrients and induced morphogenic effects in the fibroblasts and keratinocytes, resulting in greater differentiation and expression of key markers, along with increased barrier function, which was evaluated via Confocal Raman spectroscopy to analyze water and keratin content, two major contributors to the skin's barrier function. Mechanical forces applied to skin-on-chip systems not only include shear or fluid forces, but also mechanical forces, such as stretching [70]. A study by Lim et al created a human fibroblast and keratinocyte organ-on-chip model that was stretched uniaxially by 10% for 12 hours per day. This technique resulted in a wrinkled skin-on-chip (WSOC) model, and induced aging in skin-on-chip. The WSOC model exhibited decreased cell proliferation and production of collagen, fibronectin, and keratin and formed wrinkles as a result of not being able to withstand tensile stress, making it a model platform to test anti-wrinkle agents for cosmetic and medical applications.
Skin-on-chip models also offer utility as a platform to study irritation from agents that encounter the skin. Allergic contact dermatitis and irritant contact dermatitis are two common health problems, stemming from skin sensitization [71]. In addition to recapitulating the skin's physiological structure and drug response, another important component to include in a skin-on-chip model is immune competence. A model to test the allergic potential of all ingredients is of high value, as all components in a cosmetic or pharmacological product have the potential to induce a skin allergy or cause irritation. A miniaturized co-culture, with both immortalized human keratinocytes and monocytes, was combined with on-chip technology by Ramadan et al to create an immune competent skin-on-chip model. The immune cells in this model were reported to migrate and respond to skin sensitizers, modeling the interaction between keratinocytes and dendritic cells and demonstrating the potential use of this model as a tool for the study of skin sensitization and drug-induced toxicity.
3D bioprinted skin tissue is of high relevance as a technique for engineering skin constructs for wound healing [72]. Engineered skin substitutes are replacements used to aid in wound closure and improve aesthetics, especially in burn patients. Burn injuries are typically treated via autologous split-thickness skin grafts (ASSG) [72]. However, despite the success of ASSG over previous treatment methods, ASSG is limited in its capabilities. Since it is impossible to predict both the size, number, shape, and extent of a patient's burn, it is difficult to obtain donor tissue of the same parameters. Bioprinting of skin tissue may offer an alternative to ASSG, with more opportunities to personalize the tissue constructs. Bioprinting of skin tissue has been studied both in vitro and in situ, with many different cell and scaffold sources [73–83]. 3D bioprinted skin offers many advantages, such as improved personalization by incorporating patient-derived cells and enhanced plasticity and extensibility due to relevant cell organization and material patterning. However, despite its advantages, 3D bioprinting still has challenges that need to be overcome, such as availability to patients (i.e. transporting between site fabrication and clinical use site) and a lack of a vascular network for transport. Recent work by Albanna et al addresses the challenge of transporting bioprinted skin constructs to patients in clinical setting, demonstrating both design and proof-of-concept for a mobile bioprinting skin platform for on-site wound management [84]. Design criteria for the system included: (1) portability and capability to be transported quickly (2) ability to identify and measure wound size and topography accurately (3) capability to deliver multiple cell types with precise spatial orientation (4) can be easily sterilized and (5) is easily operated and has low-cost maintenance. Key components of this system include a hand-held 3D wound scanner and a printer-head with XYZ movement, mounted on a frame that is both small and mobile enough to be portable in an operating room. The developed bioprinting system combines wound scanning imaging technology with an inkjet-based bioprinter for a personalized wound treatment approach, precisely dispensing different cell types to the appropriate area. Proof-of-concept demonstrations were performed in both murine and porcine models, where the regenerated tissues had both the dermal structure and composition of healthy skin. While further studies will be needed to assess the long-term function of this bioprinted skin and expand this technology for full-thickness burns, this platform represents a breakthrough for personalized wound treatment and on-site bioprinting in a clinical setting.
An additional challenge of skin tissue engineering for clinical applications is the vascularization of engineered tissue constructs. Vascularization is crucial for long-term survival and functionality of full-thickness skin constructs, in addition to being necessary for engraftment [85]. While there are engineered skin replacements currently used in clinical settings, a competent full thickness substitute requires the ability to be vascularized [86]. As a result, complete, full-thickness substitutes are less common. The combination of bioprinting with skin-on-chip platforms may offer advantages, combining the strengths of both techniques for both improved skin tissue models and replacements. However, to our knowledge, while there have been studies combining bioprinting with organ-on-chip for other organs and tissues, there has yet to be work combining these two for skin applications. Despite the lack of work in this field, there are studies combining 3D printing with fluid perfusion, demonstrating the potential benefits of this technology pairing, such as marrying the controlled geometry of micropatterned vasculature networks, offered by 3D printing, with the improved and more efficient exchange of nutrients and oxygen, offered by a perfusion-based system. In 2016, Abaci et al published a study incorporating 3D, perfusable vascular networks in HSEs (figure 2) [85]. They used 3D printing to mold vasculature patterns, inlet and outlets for perfusion, and holders to position the vasculature within the HSEs. These 3D printed molds were then used to make sacrificial microchannels that were embedded in a dermal compartment comprised of collagen gel and dermal fibroblasts. Keratinocytes were seeded on top of the dermal compartment and ECs were seeded inside of the channels. This study was unique in that its vasculature was both micropatterned and perfusable. These components allow for use as a platform to study systemic drug delivery. We believe that this type of work is a first step in moving towards the combination of bioprinting and organ-on-a-chip for skin tissue, which will offer both the spatial control over cellular placement as is seen with 3D bioprinting and the vascular perfusion of organ-on-chip. Progress of organ-on-chip and bioprinted models of skin is summarized in table 2.
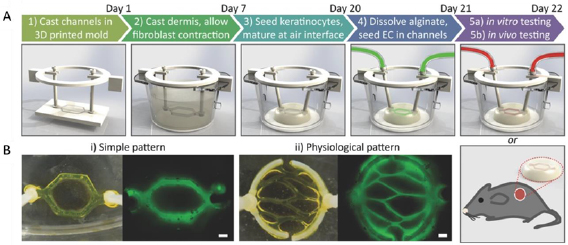
Figure 2. Development of vascularized HSEs using bioprinting and microfluidics. (A) Animated procedure for the formation of vascularized skin tissue. Sacrificial alginate was printed in the desired vasculature pattern and encased in collagen seeded with fibroblasts. Keratinocytes were seeded on top of the skin construct and the tissue was incubated to mature. The alginate was dissolved, and the hollow lumen was seeded with endothelial cells to create the dermal vasculature. (B) Photographs and fluorescent images (using fluorescently tagged alginate) of two vasculature patterns. Scale bar = 600 µm. Reproduced from [85] with permission from John Wiley & Sons. © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution imageTable 2. Summary of progress related to skin.
| Reference | Description | Major advance |
|---|---|---|
| Skin-on-chip (without bioprinting) | ||
| Abaci et al [65] | Established air-epidermal interface with a microfluidic platform for culture of human skin equivalents (HSEs) with epidermal and dermal compartments | Examined toxic effects of Doxorubicin on skin cells and structure to validate drug testing capacity |
| Wufuer et al [68] | Microfluidic device with 3 layers for co-culture of epithelial, dermal, and endothelial components | Application of tumor necrosis factor alpha (TNF-α) induced inflammation and edema, showing application for testing toxicity of cosmetics and drugs |
| Sriram et al [69] | Co-cultured human fibroblasts with neonatal immortalized keratinocytes (N/TERT) in a fibrin-based matrix combined with organ-on-a-chip | Improved epidermal morphogenesis and differentiation |
| Lim et al [70] | Fibroblast and keratinocytes perfused with media and uniaxially stretched 10% for 12 h d−1 for 7 d | Decreased production of collagen, fibronectin, and collagen suggests use as a model for skin aging |
| Ramadan et al [71] | Miniaturized immune model comprised of human keratinocytes co-cultured with a human leukemic monocyte lymphoma cell line (to model dendritic cells) | Investigation of the effect of ultraviolet (UV) and chemical treatment allowed for study of protective nature of keratinocyte layer |
| Bioprinted skin | ||
| Koch et al [73] | Laser-assisted bioprinting of fibroblasts and keratinocytes embedded in collagen | Formation of adhering and gap junctions, which are crucial for tissue morphogenesis and cohesion |
| Skardal et al [76] | Amniotic fluid-derived stem cells and bone marrow-derived mesenchymal stem cells resuspended in fibrin-collagen gel and printed over wound in mice | Increased microvessel density, capillary diameters and tracking of fluorescently labelled cells indicate that bioprinting amniotic fluid-derived stem (AFS) cells could be an effective treatment for full thickness burns and injury |
| Albanna et al [84] | Design and validation of a mobile skin bioprinting system coupled with integrated imaging technology for precise on-site delivery of fibroblasts and keratinocytes to an injury | Regenerated tissues show the dermal structure and composition of healthy skin, with collagen deposition |
| Abaci et al [85] | 3D printed HSEs with pluripotent stem cell-derived endothelial cells to form micropatterned vascular networks connected to the HSEs | Application to cutaneous wounds in immunodeficient mice demonstrates promotion of neovascularizaton during wound healing |
Bone
Bone is a metabolically active structure that supports the physical structure and integrity of the body. It maintains mineral homeostasis through bone turnover, the continuous process in which bone is formed and resorbed over a period of time. Osteocytes, osteoblasts, and osteoclasts are the main cellular components of bone tissue. Osteoblasts are located on bone surfaces and are responsible for mineralized matrix deposition. During the mineralized matrix deposition process, some of the osteoblasts are embedded in the matrix, becoming osteocytes and connecting to form networks. The counterparts of osteoblasts in bone are multinucleated cells called osteoclasts; these cells conduct the destructive part of bone turnover by resorbing mineralized bone. Bone health is an integral component to high quality of life, but the common and lethal nature of bone disease has led to the urgent need for the development of human-representative, predictive systems to aid in pre-clinical drug screening and patient treatment [15, 87]. The skeletal system is the most frequent target of metastasizing breast and prostate cancer; breast cancer patients with metastatic tumors in the bones have a five-year survival rate of only 20% [88, 89]. Osteoporosis, a 'silent' but serious disease, occurs when bone turnover is impaired and significantly increases the risk of bone fracture both in men and women over the age of 50. Several drug classes (including bisphosphonates and synthetic parathyroid hormones) were developed in the 2000s to slow bone breakdown or increase the production of new bone for osteoporosis treatment; each posed problems with long-term use, including elevated cancer risk, blood clots, and stroke [90]. The prevalence of this condition has made systems mimicking bone turnover and physiological drug response highly desirable. Emerging bone-on-chip technology has the potential to model human tissue in a more sophisticated model, improving the process of drug screening [87].
Achieving organs of relevant physiological function is no easy task [87]. The native bone consists of mineral matrix and proteins, with entrapped bone cells and vascular networks. The components of bone assemble hierarchically to provide stiffness and toughness, mechanical properties that relate to a material's resistance to deformation and ability to absorb energy without fracture [91]. Simulation of specific bone properties on a microfluidic device requires consideration of three main aspects. The first is the 3D organizational structure of bone matrix (mainly mineral and collagen), including the location of bone cells and vasculature. This aspect is fundamental for bone-on-chip technology due to the unique mechanical properties of the microenvironment [87]. The second consideration for recreating bone ex vivo is the spatially-defined patterns of soluble and matrix-embedded growth factors provided to cells. Growth factors exist in two forms, as solid and soluble molecules, and are immobilized within the ECM to cell surfaces [92]. In 2009, Phillippi et al used bone morphogenic protein (BMP) 2, combined with inkjet bioprinting, to pattern muscle-derived stem cells into spatially defined regions of osteogenic and myogenic cells. This study provided a proof-of-concept for using patterns of immobilized growth factors to spatially control differentiation of stem cells, with future implications for regenerative medicine. The final consideration for bone mimicry is the dynamic mechanical stimulations (e.g. fluid flow, strain, and hydrostatic pressure) exerted on bone cells. Bone's trabecular structure determines its load bearing capacity, and mechanical forces regulate bone cell fate, function, and bone turnover through the entire lifespan of tissue [56, 93].
Some researchers have focused on the development of bone-on-chip for cell co-culture to monitor cell–cell interactions and cancer metastasis. A microfluidic co-culture platform was developed to study osteocyte–osteoclast–osteoblast communication in the presence of mechanical stimulation [94]. Three different chambers connected by microchannels were designed to culture osteocytes, osteoclasts and osteoblasts, respectively. In this system, the osteoclasts were exposed to conditioned cell culture media from mechanically-stimulated osteocytes, and the osteoblasts were exposed to doubly conditioned media that contained soluble signals from both the osteocytes and stimulated osteoclasts. The effects of these soluble signals, from the conditioned culture media, on bone formation were quantified and compared to studies in which cells were directly stimulated mechanically. Another interesting research area is the use of microfluidic chips for the investigation of mechanotransduction in bone cells. Shear stress was applied to osteoblasts cultured on cell-matrix micro stamps, and the dynamic intracellular calcium response of a single cell was measured [95].
Bone cell activity and function are strongly dependent on the 3D microenvironment in which a large array of biochemical signals is present. Movilla et al used collagen-based hydrogels for 3D osteoblast culture in a microfluidic device and analyzed the impact of ECM properties and growth factor gradients on 3D osteoblast movement and matrix degradation [96]. Their results showed that the hydrogel mechanical properties, platelet derived growth factor β gradient, and ability of the cells to remodel the matrix affect the osteoblast's migration pattern. The 3D microfluidic approaches are also representative toolkits for modeling the dynamic microenvironment of cancer metastasis to bone. Marturano-Kruik et al developed a perfused bone perivascular niche-on-chip, using bone decellularized extracellular matrix (dECM), to support the cultivation of macroscopic bone tissues through precisely controlled flow. This device allowed live assessment of tissue development and vascularization over long periods of culture (over several weeks) and was used to elucidate the role of the bone perivascular niche in breast cancer metastatic colonization [97]. Further discussion of metastatic cancer models involving bone can be found in the 'Cancer' section below.
To make significant strides in bone-on-chip technology for drug screening, the next challenge is to establish more representative bone-mimicking micro-organoids integrated with vasculature to model a reductionist bone-on-chip [15]. Currently, 3D bioprinting technologies are mainly used to generate scaffolds replicating patient morphological features for repairing and regenerating bone defects [58]. The strategy of 3D bioprinting allows for the use of cell-laden bioinks to generate bone substitutes with in vivo-like bone tissue structures [58] and holds great potential to create models for drug screening [99]. In 2016, Cui et al used a dual bioprinting platform, comprised of fused deposition modeling and stereolithography based bioprinters, to alternately deposit polylactide fibers and gelatin methacrylate hydrogels, with encapsulated cells. Immobilized BMP2 and vascular endothelial growth factor resulted in a complex osteogenic bone construct with a vascular network, mimicking in vivo bone tissue. It is also possible to manufacture preformed vasculature in bioprinted scaffolds (figure 3) [58, 98, 100]. By exploiting the respective strengths of 3D bioprinting and microfluidics, it is possible to develop the next generation of bone-on-chip models with biomimetic reconstructions of bone tissue [98]. Despite the excellent potential of the technology, using 3D bioprinting to construct bone-on-chip systems still has several critical challenges. First, 3D bioprinted, cellularized constructs tend to have relatively low activity of embedded cells. Several approaches have been developed to increase bone cell activity, including agarose gels with incorporated bioceramic materials (polyphosphate-calcium complexes, bioglass) and nanoclay used as a bioink to increase bone cell proliferation [98, 101–103]. Second, the reconstruction of microscale scaffolds for bone-on-chip is restricted by low-resolution of bioprinting. Recent microfluidic-based strategies have facilitated the continuous fabrication of cell-laden microfibers with hierarchically organized osteon-like architecture [104, 105]. Cell-laden microfibers are mechanically rigid and provide cells with a predefined microenvironment. These fibers can define the architecture of tissue engineered constructs and can be used to recapitulate bone microstructures within microfluidic devices. Lastly, 3D bioprinting currently uses hydrogels with mechanical properties dissimilar from bone tissue. Bone tissue has peculiar characteristics as bone mineral confers high stiffness and mechanical resistance to bone tissue. Bone cell activity and function are heavily influenced by ECM stiffness [106]. Increases in the mechanical properties, such as viscosity and stiffness, of printable hydrogels must be considered. Despite the limitations discussed, the use of 3D bioprinting has great potential to close the gap between in vitro bone models and physiological bone tissues. The integration of 3D bioprinting with microfluidics is a promising technology for the construction of bone-on-chip systems. Progress in organ-on-chip and bioprinted models of bone is summarized in table 3.
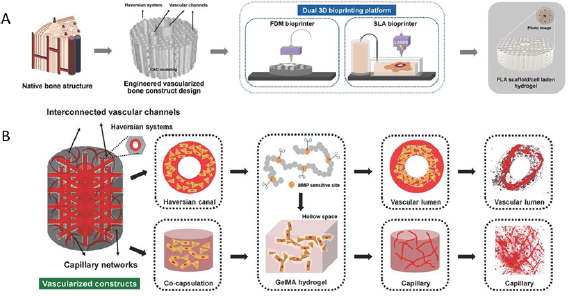
Figure 3. Fabrication of vascularized bone constructs via dual 3D bioprinting. (A) Concurrent use of fused deposition modeling (FDM) and stereolithography (SLA) bioprinting methods to generate porous, cell-laden polylactic acid (PLA) scaffolds. (B) This technology was applied to generate scaffolds with interconnected large vasculature and capillaries formed from matrix metalloproteinase (MMP)- sensitive gelatin methacryloyl (GelMA). Reproduced from [58] with permission from John Wiley & Sons. © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution imageTable 3. Summary of progress related to bone.
| Reference | Description | Major Advance |
|---|---|---|
| Bone-on-chip (without bioprinting) | ||
| George et al [94] | Seeded a modular microfluidic system with osteocytes, osteoclasts, and osteoblasts to study intercell communication through soluble factors and response to mechanical loading | Generated microfluidic devices capable of transmitting mechanical force to cells |
| Jeon et al [95] | Used a PDMS device to provide shear stress to osteoblast-like bone cells seeded on fibronectin for single cell analysis | Platform facilitated the quantification of intracellular calcium transport |
| Movilla et al [96] | Recreated biochemical gradient of platelet derived growth factor BB across a collagen hydrogel seeded with osteoblasts | Determined that osteoblast migration is heavily dependent on the cell's ability to remodel the ECM |
| Marturano-Kruik et al [97] | Seeded bone marrow MSCs and endothelial cells into decellularized bone matrix in a microfluidic device | Showed self-assembly of vasculature and bone structures in dECM |
| Bioprinted bone | ||
| Phillippi et al [92] | Patterned BMP onto fibrin-coated glass with an inkjet printer to differentiate adult stem cells into osteogenic cells | Growth factors were patterned using inkjet bioprinting for tissue engineering |
| Cui et al [58] | Used both FDM and SLA bioprinting to construct spatially heterogeneous bone scaffolds for MSC and HUVEC co-culture | Simultaneous use of two bioprinting methods to control localization of bioactive factors |
| Neufurth et al [101] | Printed osteoblast-like cells in an alginate/gelatin hydrogel coated with an agarose solution | Demonstrated the addition of an enriched agarose to induce cell proliferation and bone mineralization in a bioinert gel |
| Wang et al [102] | Incorporated bioglass into an alginate/gelatin hydrogel with osteoblast-like cells to augment mineralization | Bioglass-laden hydrogels elevated cell proliferation and calcium phosphate and carbonate mineralization |
| Zuo et al [104] | Extruded HUVEC and osteoblast-like cells into a perfusable hollow fiber using a microfluidic coaxial printing method | Osteoblast-like cells and HUVECS demonstrated upregulated gene expression and proliferation due to their 3D conformation and localization |
| Wei et al [105] | Fabricated osteon-like microfibers using a microfluidic chip extruding HUVEC and osteoblast-like cells encapsulated in RGD-modified alginate | Printed 3D structure facilitated differentiation and expression of osteogenic and vasculogenic genes |
Brain
The brain is a complex, segmented organ that is responsible for controlling the functions of the body by neural activity in distinct regions [107]. Microfluidic models of the human brain aim to study a vast array of phenomena including microenvironmental conditions, physiological interfaces, electrophysiological properties, and disease states. Comprehensive reviews of these microfluidic devices are available from Yi et al [108] and Zhuang et al [109]. Many microfluidic devices have incorporated microelectrode arrays that are useful for studying electrical activity in brain slices and neuronal networks. These devices have also been used to study the progression of neurodegenerative diseases (such as Alzheimer's and Parkinson's Disease) and the injury and repair of severed axons. This section covers the commonly modeled physiological structures of the brain and central nervous system including the blood-brain barrier (BBB) and nerves stemming from the brain and spinal cord.
The BBB is a well-studied physiological structure in the brain due to its relevance to drug delivery and toxicity [110, 111]. The barrier involves a vascular endothelial layer that overexpresses tight junctions to restrict permeability of blood-borne molecules into the surrounding brain tissue [112]. This endothelial layer directly interacts with astrocytes and indirectly with pericytes and neurons. Extensive work has been published regarding devices mimicking the BBB and several reviews of these systems are available [110, 111, 113–115]. One model of the BBB filled parallel channels with collagen hydrogels containing astrocytes and neurons next to an endothelium-lined channel [116]. This device maintained the cell-specific morphology and function for each cell type. Similar work has aimed to increase physiological relevance by introducing reprogrammed iPSCs to obtain brain microvasculature ECs [117] and perfusing cell-type specific media to maintain cell properties [118]. A recent advance is the incorporation of microvasculature, self-assembled through a co-culture of endothelial cells, astrocytes, and pericytes in a fibrin hydrogel [119]. Gene expression was studied in the presence and absence of the other cell types. The group demonstrated that co-culture with each cell type significantly increased gene expression in each cell type, suggesting that the device allowed the study of active and representative cells in the device. In addition to systems aiming to maximize physiological relevance, some devices have been designed with scalability and commercial use in mind. Jeong et al developed an array of BBB interfaces using neural ECs and astrocytes [120]. In addition to recreating the barrier, the device incorporated electrical sensors to analyze the connectivity of cells in the endothelial layer; integration of sensors to gather quantitative data in real time is an important aspect of developing devices suitable for commercial use. Interestingly, Emulate, Inc. and Cedars-Sinai Medical Center were recently granted a patent on a BBB device to co-culture endothelial cells, astrocytes, and neurons [121]. While the device does not have the complexity of some other models, it demonstrates that commercial applications require simple, yet representative, systems. Each of these models was validated by the presence of tight junctions, transendothelial electrical resistance (TEER) assays, and analysis of molecular weight selectivity of the barrier. While BBB models alone can provide limited information on the efficacy and safety of drugs, devices integrating the barrier into multi-organ chip devices [122] can produce models that better represent efficacy and toxicity of drugs.
Another type of microfluidic device used to study the brain and nervous system aims to isolate the axon of a neuron from the cell body. Models that isolate the axon are imperative in understanding the cellular processes that occur during signal transduction [123] as the axons of a neuron can extend up to one meter and the microenvironment near the axon terminals will likely differ greatly from that of the cell body and dendrites [124]. The original design for the microfluidic device was published in 2005 by Taylor et al; the system connects two parallel channels 100 µm deep with perpendicular 3 µm channels [125]. While the axons from the neuron can grow through the small channels, fluid cannot be exchanged. This creates two distinct microenvironments: one for the cell body, and another for the terminal axons. Since 2005, this design has been integrated with microelectrode arrays to study the electrophysiology along the length of the axon [126, 127]. This device design is also optimal for studying the generation and repair of axons. A 2010 study conducted by Hellman et al damaged axons using a laser [128]. They then studied the effect of egtazic acid (EGTA), a calcium chelator, on the ability of the axon to resist death and induce repair. They concluded that the sequestration of calcium reduced the extent of axonal injury but slowed the repair of the cell. Further studies with similar methods may provide methods of treating patients with traumatic brain injury or nerve damage.
Several devices have been adapted to study a variety of neurological disorders such as Alzheimer's and Parkinson's Disease. Review articles discussing the use of microfluidics for studying disorders of the central nervous system have been published by Yi et al [108] and Jorfi et al [129]. Recent research has featured an axon-isolating microfluidic device used to study the production and effects of amyloid beta (Aβ) peptides on both the axon terminals and the neuronal body [130]. This study concluded that Aβ peptides were secreted more by the axon compared to the dendritic cell body and hopes to provide a platform for the development of therapeutics based on the new knowledge of amyloid plaque production. A different device was used to study degeneration and inflammation in a 3D co-culture of neurons, astrocytes, and microglia in an Alzheimer's disease environment [131]. The authors were able to recreate the phenomena of microglial recruitment, axonal cleavage, and nitric oxide-induced cell damage. Beyond Alzheimer's disease, systems have been developed for studying Parkinson's disease. One model demonstrated that the addition of α-synuclein induced the formation of Lewy Bodies (pathogenic protein aggregates) which were then propagated amongst neuron bodies to spread disease [132]. A similar device was also used to observe mitochondrial transport across the axon, a phenomena associated with Parkinson's disease [133]. Microfluidic devices have also been used to study amyotrophic lateral sclerosis (ALS) [134], stroke [135], multiple sclerosis (MS) [136], migraines [137], and epilepsy [138].
Use of 3D in vitro neural tissue models recapitulate cell–cell and cell–ECM interactions with a much higher fidelity than 2D culture models [109]. The emergence of bioprinting presents a strategy for creating improved 3D neural tissue models. One application of bioprinting for CNS models is to create neuronal tissues to replace existing 2D and 3D culture systems. In 2018, De La Vega et al bioprinted neural tissue by 3D printing human iPSC (hiPSC)-derived neural progenitor cells (NPCs) (figure 4) [139]. Their printing procedure resulted in high cell viability (~81%) and improved differentiation capacity, compared to previous bioprinting strategies [139]. Results showed that these bioprinted neural tissues expressed neuronal markers, spinal cord motor neuron markers, and mature motor neuron markers, demonstrating this strategy's potential as a high-throughput technique for producing hiPSC derived neuronal tissue [139].
Figure 4. Current progress in the microfluidic modeling and bioprinting of neural tissue. (A) Microfluidic platform for modeling the blood brain barrier (BBB). (i) Animation of a perfusable vascular network interacting with astrocytes and neurons in a microfluidic device. (ii) Steps in the self-assembly of the BBB in the perfusable chip. Reproduced from [118]. CC BY 4.0. (B) (i) Schematic representation of Lab-on-a-Printer (LOP)™ printhead and bioprinted neural tissue construct. (ii) Bright field microscopy image of the bioprinted construct consisting of human induced pluripotent stem cell (hiPSC)-derived neural progenitor cells (NPCs) and a fibrin-based bioink. Scale bar represents 3 mm. Reproduced from [139]. CC BY 4.0.
Download figure:
Standard image High-resolution imageAnother interesting application of neural tissue bioprinting is drug development and screening. The identification and validation of drugs for the treatment of Alzheimer's disease is an extremely challenging and expensive process [140]. As the occurrence rate of Alzheimer's disease increases, the need to identify more efficacious drug targets becomes more pressing [140]. Current models and tools for drug identification are lacking and ineffective. To advance the treatment of Alzheimer's, and other diseases, we must develop better strategies to screen drugs before they reach clinical trials. This improvement will save resources, time, and reduce the cost of drug development. 3D bioprinting of neural tissues can be used as a tool for the screening of potential drug candidates and targets [140]. A recent report predicts that 3D bioprinting may provide a solution and serve as an alternate platform for screening and developing Alzheimer's drugs, providing a solution to a progressing problem [140]. This prediction is based on recent research studies [141] and the work summarized in recent reviews [142]. In 2017, Gu et al developed a bioink for the printing and subsequent differentiation of iPSCs [141]. After printing the iPSCs, the authors waited 3 days and then cultured the printed cells in neural induction media for 2–3 weeks, followed by differentiation via media with brain-derived neurotrophic factor [141]. Immunophenotyping was used to confirm the presence of neural markers. The ability to print iPSCs and then differentiate the printed cells into specific lineages presents a huge step in the development of models for the study of disease and for drug development and screening [141].
To our knowledge, there have not been studies that combine 3D bioprinting and organ-on-chip for the creation of neural tissue models. However, despite the scarcity of research in this space, we predict that these two technologies, when combined, may generate improved models. As discussed above, on-chip CNS models provide improved physiological relevance and recapitulate crucial features of the cell microenvironment, such as perfusion, as well as allowing for advanced features, such as sensor integration, which vastly improve data collection. On the other hand, 3D bioprinting provides a fast, accurate method for creating complex neural tissues. In the future, more advanced models, such as that for Alzheimer's study and drug development, will emerge. Such studies will greatly contribute to our understanding of the CNS and provide improved preclinical models, improving healthcare greatly. Progress in organ-on-chip and bioprinted models of brain is summarized in table 4.
Table 4. Summary of progress related to neural tissue.
| Reference | Description | Major advance |
|---|---|---|
| Neural tissue-on-chip (without bioprinting) | ||
| Ma et al [111] | Astrocytes and endothelial cells were cultured on either side of nanofabricated porous silicon nitride membranes | Despite observing contact through large membrane pores, the membranes failed to beat commercially available alternatives in forming ultra-tight junctions |
| Adriani et al [116] | Co-cultured astrocytes and neurons seeded in adjacent collagen matrices next to an endothelial cell-lined channel | Observed restrictive endothelial barrier similar to BBB and neuronal outgrowth through collagen |
| Wang et al [117] | Cultured astrocytes and induced pluripotent stem cell (iPSC)-derived brain microvascular endothelial cells (BMECS) on opposite sides of a porous membrane in a pumpless microfluidic device | Near in vivo permeability was observed through transepithelial/transendothelial electrical resistance (TEER) and molecule permeability analysis |
| Bang et al [118] | Vasculature self-assembled in a fibrin matrix with adjacent neurons and astrocytes forming the blood brain barrier (BBB) | Barrier function was improved by supplying each cell type with a corresponding optimized media |
| Campisi et al [119] | Co-cultured astrocytes, pericytes, and iPSC-derived endothelial cells in a fibrin matrix | Observed self-assembly of vasculature and physiologically representative selective BBB |
| Jeong et al [120] | Integrated electrical sensors to perform on-chip TEER measurements across an astrocyte-endothelial interface along a polycarbonate membrane | Designed chip to have integrated analytical methods to study barrier function |
| Kerns et al [121] | Patented a device containing BMECSs cultured on a membrane | Demonstrated commercial interest in applying organ-on-chip systems for patient care |
| Pinto et al [123] | Used axon-isolating chip to study the ubiquitin-proteasome system | Proteasome inhibition facilitated synaptogenic response by axons |
| Taylor et al [125] | Seeded neurons in one channel and allowed axons to pass through 3 µm channels to a secondary media reservoir | Allowed the isolation of the neuronal cell body from the terminal axons |
| Habibey et al [126] | Adapted axon-isolating chip design for integration with microelectrode arrays aligned with the axon channels for signal transduction monitoring | Integrated electrical sensors for real-time signal monitoring |
| Moutaux et al [127] | Incorporated axonal and postsynaptic electrodes into an axon-isolating microfluidic device | Used in tandem with video processing to understand intracellular dynamics of signal transduction |
| Hellman et al [128] | Used a laser to induce axonal injury in an axon-isolating microfluidic chip | Observed that calcium sequestration reduces the extent of cell injury, but also slows cellular repair |
| Li et al [130] | Adapted axon-isolating device to create chemotactic gradients for studying neuronal production and susceptibility to amyloid beta plaques | Results suggested a localized mechanism for the production of, and toxicity to, amyloid beta plaques |
| Park et al [131] | Neurons, astrocytes, and microglia seeded in Matrigel were maintained in a microfluidic device to recreate an Alzheimer's disease microenvironment | Hallmarks of the disease, such as microglial recruitment, axonal cleavage, and nitric oxide-induced damage, were recapitulated in vitro |
| Volpicelli-Daley et al [132] | Adopted axon-isolating chip to study the creation of Lewy bodies resulting from alpha-synuclein | Used to emulate Parkinson's disease in vitro |
| Lu et al [133] | Used axon-isolating chip to observe transport of mitochondria in axon | Imaged mitochondrial transfer, a component of neurodegenerative disease |
| Kunze et al [134] | Performed astrocyte-neuron co-culture in axon-isolating device | Observed decreased neuron viability in the presence of mutant astrocytes mimicking amyotrophic lateral sclerosis (ALS) |
| Samson et al [135] | Modeled stroke damage in an axon-isolating culture model | Demonstrated that networks of neurons can help inhibit spreading of excitotoxic signals |
| Hosmane et al [136] | Co-cultured neurons and microglia in an axon-isolating device to develop nerve bundles as a model of nervous system injury | Elucidated the mechanism of signaling leading to the phagocytosis of axon terminals |
| Tang et al [137] | Developed a device to perfuse brain slices and deliver local chemical stimuli | Developed a method of localizing chemical stimulation to a region of a brain slice |
| Hill et al [138] | Analyzed brain slices laid across a multi-electrode array | Used system to study the origin and propagation of epileptiform brain signals |
| Bioprinted neural tissue | ||
| De la Vega et al [139] | Used a microfluidic bioprinting method to print iPSC-derived NPCs in a fibrinogen/alginate bioink | Method yields 3D structures of iPSC-derived neural tissue |
| Gu et al [140] | Uses extrusion-based bioprinting to construct scaffolds composed of iPSCs seeded in an alginate-agarose-carboxymethyl chitosan bioink | Exhibited the ability to yield porous homogenous structures of migratory neurons and neuroglia or the formation of embryoid bodies composed of each of the three germ layers |
Lung
The human lung serves as an interface between the human body and the environment, acting as an entry portal for vapors, airborne particles, and aerosols. Epithelial tissue barriers protect the body from allergens, air contaminants, and infectious agents. Despite the presence of these tissue barriers, incidence of respiratory diseases are still prominent ailments [143, 144]. In the last few years, researchers have attempted to elucidate the mechanisms through which antigens avoid these barriers; therefore, many alternative cellular models have been established [145]. Lung-on-chip systems allow for more precise control of the cellular microenvironment and recapitulate in vivo biochemical contexts better than other in vitro models, or even traditional in vivo animal models.
In one study, Huh et al developed a multifunctional microdevice that reconstituted responses to bacterial and inflammatory cytokines, on the organ level, as well mechanical activity on a chip [146]. This was accomplished using two side-by-side microchannels, filled respectively with alveolar epithelial and microvascular ECs, separated by a thin porous membrane. Using vacuum chambers, they performed cyclic stretching on the interface, simulating the motions of breathing. In their device, the cells remained viable for more than two weeks. The incorporation of air in the upper chamber increased the production of surfactant by the epithelial cells, stabilizing the thin liquid layer, as is seen in vivo, eliminating drying. In addition, the authors also identified an increase in the electrical resistance across the two tissue layers and an improvement in the function of relative barrier function, compared to cells cultured in traditional 2D platforms. The cyclic strain application also induced alignment in the cells in the endothelial layer and thereby mimicked the physiological responses previously observed in living blood vessels. The stretching system integrated into the device is unique as it allowed cyclic mechanical stimulation while fluid shear stress was applied to two cell layers. Additionally, this stimulation mechanism did not interfere with the analysis of barrier function (permeability and transport) in the tissue.
More recently, Humayun et al reported a thermoplastic, microfluidic lung-on-chip model that captured both the microenvironment of lung tissue and the interactions between SMCs, ECs, and supporting ECM [147]. The device was constructed from acrylic and consisted of three vertical microfluidic chambers: a lower reservoir containing media for SMC culture, a middle layer of hydrogel, and an upper chamber to achieve air–liquid interface epithelium culture. A Matrigel and type I collagen mixture was found to promote adhesion and monolayer formation in ECs, as well as SMC adhesion and alignment. The primary utilities of the device were long-term SMC–EC co-culture, the ability to perform immunofluorescence staining on chip, the ability to handle samples without disruption of the matrix or cells, and the ability to perform high-throughput studies.
In 2018, Wang et al developed an improved in vitro model of lung cancer using low-temperature molding and 3D bioprinting [148]. With these two biological manufacturing techniques, they fabricated a hydrogel grid scaffold using a suspension comprised of gelatin-sodium alginate and lung cancer cells. The cells in this scaffold were homogenously distributed and exhibited high viability and culture sustainably. The results showed that this model could be cultured and maintain its structural integrity for 28 days. Moreover, histology, gene analysis, and scratch testing showed enhanced invasion and migration capability in the 3D printed cells, when compared to 2D cultured cells. Cell viability after the printing process remained over 90%, showing that the temperature and pressure changes the cells encountered during the 3D printing process caused little damage. Additionally, cells in this model had more cell–cell contacts than 2D cultured cells, indicating increased intercellular interactions and communication, as observed by scanning electron microscopy. They observed no significant differences between the 3D bioprinted and 2D cultured cells, for both the A549 and 95-D cell lines, after 28 days. However, for both cell types, after 8 days, the proliferation rate in 3D culture increased and in the 2D cultured cells declined. Cells cultured in 3D had significantly increased proliferation, while the 2D cultured cells declined after 8 days. The proliferation rate of the 3D cultured cells reached a maximum peak from day 12 to 14, declining after the 14th day. This microdevice successfully reconstituted key features of the alveolar-capillary interface in the human lung and provides the groundwork for further advances to form a larger working model.
3D bioprinting enables automated fabrication of tissue structures and facilitates precise deposition of cells, mimicking the cellular arrangement of native tissue [149]. By using bioprinting tools, Horváth et al engineered an in vitro lung-on-chip [150]. The human air-blood barrier analogue system was created using a layer-by-layer 3D printing approach and was comprised of alveolar epithelial type II and endothelial cells, separated by a thin membrane matrix. The goal of this work was to use valve-based bioprinting to fabricate a complex 3D air-blood tissue barrier in a layer-by-layer process. The resulting engineered lung tissue closely recapitulated the human air-blood barrier. The authors performed comparative studies between manually seeded and bioprinted cultures, looking at cellular morphology and intercellular contacts. The results demonstrated the production of an optimal air-blood tissue barrier construct and an optimized, reproducible fabrication technique.
Furthermore, to improve the reproducibility of bioprinted lung-on-chip fabrication, one-step 3D cell printing processes have been developed, creating platforms with complex 3D cellular structures [151]. Following this approach, Park et al fabricated a vascularized lung-on-chip via 3D cell printing (figure 5) [152]. The integrated platform developed was comprised of a 3D printed vascular platform of cell-laden ECM bioinks, integrated with an airway epithelium model. This platform was used to recapitulate inflammatory response and simulate pathophysiology. This lung-on-chip faithfully recapitulated functional lung tissue microstructure, along with its vascular network, reinforcing the promising applications of 3D bioprinted lung-on-chip and motivating further study. Progress in organ-on-chip and bioprinted models of lung is summarized in table 5.
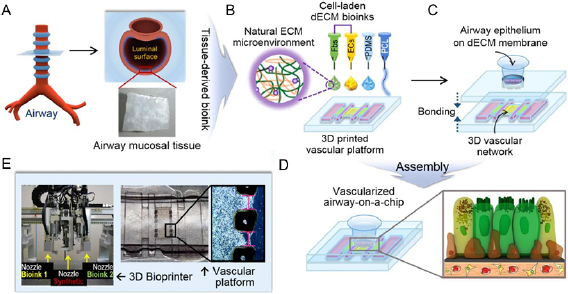
Figure 5. A functional airway-on-chip produced using 3D bioprinting. (A) Decellularized airway mucosal tissue was harvested from the luminal surface of the trachea. (B) The harvested tissue was used to create a printable bioink seeded with fibroblasts and vascular endothelial cells. (C) Airway epithelial cells seeded on a decellularized extracellular matrix (dECM) to create an interface between the tracheal epithelium and vascular endothelium. (D) Animation of the interface between fibroblasts, vascular endothelial, and airway cells on a dECM membrane. (E) A multi-head bioprinter was used to load the microfluidic device with the cell-laden bioinks. Reproduced from [152]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageTable 5. Summary of progress related to lungs.
| Reference | Description | Major advance |
|---|---|---|
| Lung-on-chip (without bioprinting) | ||
| Huh et al [146] | Constructed a four-chamber microfluidic device with an alveolar epithelium and vascular endothelium interface | The mechanically active device recapitulated in vivo response to bacterial insult and inflammation |
| Humayun et al [147] | Generated a blood-air interface by seeding endothelial and SMCs on either side of a collagen/Matrigel suspended hydrogel | Enabled cell maintenance for more than 31 d with expression of in vivo morphological markers of ECs and SMCs |
| Bioprinted lung | ||
| Wang et al [148] | Used low-temperature molding and 3D bioprinting to create a gelatin/sodium alginate hydrogel seeded with lung cancer cells | Demonstrated that the 3D lung cancer cells were more prone to migration and invasion than their 2D analogs |
| Horváth et al [150] | Bioprinted a Matrigel-separated bilayer of endothelial and lung ECs in a transwell dish | Created homogenous cell layers through automated 3D bioprinting to mimic the air-blood barrier |
| Lung-on-chip (with 3D bioprinting) | ||
| Park et al [152] | Printed endothelial cells seeded in tracheal mucosa extracellular matrix (ECM) in a polycaprolactone (PCL) device | Native ECM drove the self-assembly of a vascular network and recreated asthamatic inflammation in vitro |
Heart
The heart is a vital organ that pumps blood through the vascular system to nourish the tissues and organs of the body and maintain homeostasis. Worldwide, heart disease is the leading cause of death in both men and women [153]. Heart disease encompasses a variety of cardiovascular conditions and usually can be managed with lifestyle changes, medication, or implantable medical devices [154]. Severe heart diseases are resistant to typical treatment methods and may require surgery to repair defects or transplant the heart altogether and, like other transplantable organs, demand greatly exceeds supply [155]. Due to the ubiquity of the problem, a tremendous effort has been made to engineer functional cardiac tissue for heart regeneration [156–159].
The heart also has implications in the development and prescription of drugs. Approximately one third of safety-based pharmaceutical withdrawals are due to cardiotoxicity [6, 7]. Cardiotoxicity is a great concern as it can lead to arrythmia and eventual heart failure. These abnormalities are especially common with anti-cancer drugs [160, 161]. Conventional 2D in vitro and animal models do not adequately mimic the biology and physiology of the human myocardium [162]. Animal models are insufficient for cardiotoxicity studies as they are expensive, time-consuming, and unrepresentative of drug response due to interspecies variation [163, 164]. The lack of representative models of drug response has created an urgent need for high-throughput in vitro systems that can predict the physiological response to therapeutics.
Recent progress in engineered stem cells, biocompatible scaffolds, and in vitro tissue vascularization has facilitated the development of representative cardiac tissue. These advances have generated progress in the development of physiologically relevant microfluidic human heart models for applications in drug discovery and personalized medicine. A microfluidic heart-on-chip device addresses some of the drawbacks of 2D cell culture and animal models by recreating the topological, electromechanical, and biochemical features of native tissue to better mimic in vivo behavior [159, 165, 166]. These devices are integrated with perfusable microfluidic networks simulating physiological fluid flows. These not only distribute nutrients and collects waste, but also contributes to the maturation of cardiac tissue by delivering soluble biomolecules [159, 167].
Mathur et al presented a cardiac microphysiological system (MPS) to predict cardiotoxicity by incorporating human-derived cells with physiologically relevant alignment and tissue structure into a device [162]. The system simulated human vasculature and facilitated biological, electrophysiological, and physiological analysis. Their MPS model was able to maintain functional human iPSC-derived cardiac tissue over several weeks. A cardiac organoid was recreated by culturing cardiomyocytes in a central channel with an array of microposts simulating the endothelial barrier. This barrier reproduced the in vivo fluid dynamics and diffusive transport of nutrients and waste by creating a restrictive, shear-shielding barrier between the media flow and muscle tissue.
Another device created by Marsano et al focused on mimicking the mechanical microenvironment experienced by cells in the myocardium [168]. The cell-laden gel was contained by an array of hanging posts and cyclic strain was induced on the crosslinked tissue using a pneumatic system. This system was able to generate functional and mature micro-engineered cardiac tissues from neonatal rat and human iPSC-derived cardiomyocytes. The mechanically-stimulated tissues showed increased cardiac differentiation and higher expression of junction proteins leading to better mechanical and electrical conductance.
Agarwal et al developed a high-throughput heart-on-chip to monitor the contraction of muscle filaments [169]. The group semi-automated the fabrication process to create a scalable array of sub-millimeter thin film cantilevers. Cardiomyocytes were cultured on the cantilevers to create anisotropic cardiac microtissues that mimic heart ventricles. The cantilever deflection as a result of the muscle tissue contraction can be quantified to measure the diastolic and systolic stresses generated by the tissues. This device was used to observe the dose-dependent impact of isoproterenol on cardiac contractility. An interesting aspect of this device is the emphasis on manufacturing methods compatible with large scale production and commercial use. The design has one channel and is constructed from autoclavable materials that allow optical evaluation of cardiac contractility; additionally, the metallic base is designed for temperature control and electrical stimulation.
Cardiac tissue engineering has also benefitted from modern advances in 3D bioprinting. A 2012 study by Gaetani et al demonstrated the viability of bioprinting with human cardiomyocyte progenitor cells in alginate scaffolds [170]. They demonstrated that the cells remained viable for one week and that the culture conditions did not significantly modify growth and phenotypic commitment. This model can be used to study variability between 2D and 3D cell culture models in addition to the effects of the ECM composition and presence of small molecules in the microenvironment.
3D bioprinting, microfluidics, and stem cell technology were integrated to create an endothelialized-myocardium-on-chip (figure 6) [171]. Coaxial bioprinting was used to create endothelialized myocardial fibers by first printing ECs inside microfibers. Over time, the cells migrated to the outside of the fibers and formed a consistent layer on the surface and the structure was seeded with cardiomyocytes. The resulting semi-self-assembled structure accurately recreated the in vivo, vascularized structure of the myocardium. This manufacturing approach, combined with a microfluidic perfusion bioreactor, was applied as a platform for cardiovascular drug screening. The team observed dose-dependent responses to an anti-cancer drug, doxorubicin, from both cardiomyocytes and ECs. This platform demonstrates the synergistic potential of fluidics and 3D bioprinting towards the development of the next-generation of human organ models. Advanced organ-on-chip systems can emulate healthy and diseased myocardial tissue. In the context of personalized medicine, their predictive ability shows potential to mitigate drug-induced cardiovascular toxicity and improve treatment efficacy [171–174].
Figure 6. Fabrication steps for 3D bioprinting of endothelialized myocardium. Schematic illustrating the fabrication protocol for endothelialized myocardium using a 3D bioprinting strategy. First, a hydrogel fiber encasing endothelial cells was bioprinted to create a 3D scaffold. The vascular bed was formed by endothelial cell migration to the exterior of the fiber. Cardiomyocytes were seeded on the exterior of the fibers to create the endothelialized myocardium. Reproduced from [171]. Copyright 2016, with permission from Elsevier.
Download figure:
Standard image High-resolution imageDespite the success of integrating the two techniques, current microphysiological systems are restricted by the lack of integrated analytical methods and complex, multi-step fabrication processes. Lind et al proposed a device that surmounts these challenges by using multi-material 3D bioprinting technology to include gauge wire for real-time quantitative measurements of cardiac tissue [174]. The 3D printing method utilized six inks, each with a different purpose. Some inks, such as dextran, thermoplastic polyurethane, and shear-thinning PDMS, served as substrates and self-assembly cues, while others, such as the carbon black in thermoplastic polyurethane and silver-laden polyamide, composed the soft electrical components of the system. The six inks were used to create three distinct components (the base, the sensor, and the tissue-guiding layer) used to support, measure, and manipulate the cells on the surface. This microphysiological device facilitated tissue culture and the non-invasive analyses of tissue contractile strength over several weeks. The ability to sustain functioning tissues facilitates long-term drug studies in a controlled ambient environment. After validating the effective performance of the embedded sensors inside a cell culture incubator, the system was used to measure contractile stress in relation to administration of isoproterenol and verapamil. A strong dose-dependent response to both drugs was observed. In addition to drug testing, the contractile development of human stem cell-derived laminar cardiac tissues was tracked over the course of four weeks. Progress in organ-on-chip and bioprinted models of heart is summarized in table 6.
Table 6. Summary of progress related to cardiac tissue.
| Reference | Description | Major advance |
|---|---|---|
| Heart-on-chip (without bioprinting) | ||
| Annabi et al [167] | Coated polydimethyl siloxane (PDMS) microchannels with gelatin methacryloyl (GelMA) and MeTro to facilitate adherence of primary cardiomyocytes to study cell beating | Identified tropoelastin as a suitable hydrogel for mimicking in vivo soft substrates |
| Mathur et al [162] | Cultured cardiac tissue derived from human induced pluripotent stem cells (hiPSCs) in a perfusable microfluidic chamber | Sustained beating human cardiomyocytes for several weeks in vitro, demonstrated similar pharmacological response compared to analogous tissue assays |
| Marsano et al [168] | hIPSC-derived cardiomyocytes seeded in fibrin gel were mechanically stimulated in a PDMS microfluidic device | Mechanical stimulation promoted cardiac differentiation, maturation, and mechanical and electrical coupling; system was used to observe cardiotoxicity of isoprenaline |
| Agarwal et al [169] | Created muscular thin films composed of cardiomyocytes patterned on PDMS cantilevers within a fluidic device | Device facilitated optical characterization of heart tissue contractility |
| Bioprinted heart | ||
| Gaetani et al [170] | Fetal cardiomyocyte progenitor cells were printed in a sodium alginate hydrogel for cardiac tissue engineering | Printed progenitor cells remained functional and committed to their cardiac lineage, cells showed enhanced expression of early cardiac transcription factors in 3D culture |
| Heart tissue-on-chip (with bioprinting) | ||
| Zhang et al [171] | Perfused a 3D lattice composed of coaxially printed, endothelial-seeded, cardiomyocyte-covered GelMA/alginate fibers in a microfluidic chamber | Generated perfusable, endothelialized cardiac organoids capable of modeling the native myocardium for drug-related cardiotoxicity assays |
| Lind et al [174] | Utilized a multi-ink extrusion printer to manufacture a fibronectin-coated micropatterned substrate to measure the contractility of ventricular myocytes and iPSC-derived cardiomyocytes | Integrated metallic contractility sensors into the organ-on-chip device using 3D printing |
Liver
The liver is a major site of drug metabolism and filtering in the body [175]. As a result, there is a high incidence rate of drug-induced injury to the liver. As previously discussed, there is a need to improve in vitro pre-clinical models, as current in vitro models do not fully recapitulate human physiology and in vivo models are often inaccurate and costly. As is the case with other human organs, a model to study hepatotoxicity would be of great utility [176]. However, one unique feature of the liver is its ability to regenerate [175]. The liver's plasticity, along with other aspects, drive the need for liver-on-chip models, for the purpose of both the study of the liver system and the study of drug development for improved screening [175].
Many studies have investigated the ability of these models to recapitulate fundamental features of the liver and their physiology, as well as their response to known pharmacological compounds. In 2017, Yu et al performed a study to work towards the development of liver-on-chip to assess chronic hepatotoxicity caused by repeated drug dosing [177]. As a control, they demonstrated that constant hepatic function (urea, albumin synthesis, and CYP450 enzyme activities) could be maintained over two weeks at constant values, as has been achieved by many groups previously, demonstrating the ability of their model to recapitulate liver functionality [177]. They designed a perfusion-incubator-liver-chip, with tangential flow over the cultured spheroids [177]. The results show a more sensitive response to Diclofenac and Acetaminophen than static culture, in contrast to previous in vitro studies where only high concentrations of Diclofenac induce toxicity, representing an advance in the recapitulation of the liver [177]. However, while this liver-on-chip platform mimics in vivo liver drug response to a singular drug with high fidelity, in order to successfully model liver toxicity, a platform must well model native responses to many classes of drugs.
A unique application for liver-on-chip technology has emerged in the food safety industry [178]. In 2017, Nature News published a piece on work by the Food and Drug Administration (FDA) with a liver-on-chip application to model human reaction to foods and food-borne illnesses [178]. While the chips used were originally developed for the purpose of drug testing, these miniaturized liver organs can test the effect of food-borne pathogens on humans, as well as the effect of dietary supplements and cosmetics on human organs [178]. This work with the FDA represents a new use for organ-on-chip technologies, demonstrating the necessity for regulatory agencies to explore alternatives to in vivo animal testing and offering promise as an improved alternative to subpar models [178].
Bioprinted tissues offer many advantages over other approaches to tissue engineering. One such advantage is the integration of both the cells and scaffold; bioprinting co-prints the scaffold material, and the cells, unifying the manufacturing process. In 2016, a drug induced toxicity study was performed on 3D bioprinted primary liver tissues [179]. This model sought to overcome the limitations of existing liver models, including the lack of longevity and tissue-level complexity observed in previous 2D monocultures [179]. This study combined patient-derived primary cells and bioprinting and is a large improvement in models for drug toxicity [179]. Additionally, work in 2016 by Norona et al demonstrates the utility of bioprinted liver for detecting several kinds of liver injury, i.e. hepatocellular damage and fibrogenesis [180]. The data collected from this study demonstrates the ability of 3D bioprinted liver tissues to recapitulate drug and chemical fibrogenesis at the multiple levels (cellular, molecular, and histological), providing a mechanism to further study liver fibrogenesis, a process that occurs during the onset and progression of liver damage [180].
Another advantage of bioprinting is the capability to create 3D structures with channels for perfusion, improving nutritional supply to a large number of cells, overcoming a major challenge of spheroid-type cultures [181]. In 2018, Grix et al presented a proof of concept study for the bioprinting of liver organoids, using a stereolithographic printing approach and HepaRG cells, as well as human stellate cells [181]. Evidence of the perfusability of the channel system was provided via metabolite analysis, motivating further development of organoid bioprinting [181].
Important advances in drug screening models combine bioprinting with microfluidic platforms for improved tissue models [176]. One such example of a bioprinted liver-on-chip included hepatic spheroids, which are encapsulated in a hydrogel scaffold in a microfluidic platform (figure 7) [182]. This platform allowed for long-term culture of 3D HepG2/C3A spheroids for the assessment of liver toxicity [182]. The 3D hepatic spheroids were encapsulated within photocrosslinkable gelatin methacryloyl (GelMA) and functioned for 30 days [182]. Proof of concept experiments were performed, demonstrating a toxic response to 15 mM of acetaminophen that was similar to previously reported in vivo animal responses, indicating much promise for this platform as a tool for assessment of drug-induced liver toxicity [182]. The combination of bioprinting and liver-on-chip technology offers additional capabilities in controlling cell composition and 3D structure that will be necessary to make the next generation of in vitro liver models. Progress in organ-on-chip and bioprinted models of liver is summarized in table 7.
Figure 7. A microfluidic liver-on-chip produced using 3D bioprinting. (A) Bioprinting hepatic tissue into a perfusable liver-on-chip bioreactor culture platform. (ii) (a) Bioprinting a (b) dot array of photocrosslinkable gelatin methacryloyl (GelMA)-based (c) hepatic spheroids. Scale bar = 200 µm. (iii) (a) Top-view and (b) side-view of the assembled bioreactor with labeled inlet and outlet fluidic ports. Scale bar = 1 mm. Reproduced from [182]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageTable 7. Summary of progress related to liver tissue.
| Reference | Description | Major advance |
|---|---|---|
| Liver-on-chip (without bioprinting) | ||
| Yu et al [177] | Rat hepatocyte spheroids constrained between a cover glass and a porous, ultrathin Parylene C membrane experiencing flowing culture media | Response to repeated dosing of Diclofenac and Acetaminophen more sensitive than static culture control |
| Bioprinted liver | ||
| Nguyen et al [179] | Printed scaffold-free liver tissue mimetics, comprised of patient-derived hepatocytes and non-parenchymal cells | Maintained levels of ATP, Albumin, and drug-induced Cytochrome P450s activity for 4 weeks in culture |
| Norona et al [180] | Bioprinted tissue comprised of primary hepatocytes, hepatic cells, and endothelial cells exposed to low dose of methotrexate thioacetamide repeatedly | Detected multiple modes of liver injury, including hepatocellular damage and fibrogenesis |
| Grix et al [181] | Stereolithographic printed liver organoids with HepaRG and human stellate cells | Tight junctions, liver-specific bile transporter multidrug resistance-associated protein 2 (MRP2), and overall metabolism provide evidence for perfusability of organoid's channel system |
| Liver-on-chip (with 3D bioprinting) | ||
| Bhise et al [182] | Human HepG2/C3A spheroids in GelMA within a chip platform for 30 d culture | Utility as a platform for testing drugs demonstrated by showing similar response to treatment with 15 mM acetaminophen as animal models |
Gut
The gut plays an essential role in the digestion of food and absorption of nutrients into the body. Like many other organs, there has been significant effort devoted to the development of in vitro models recreating the structure and function of the intestines. The hallmarks of the gut are its crypt-villus structures that line the lumen of the intestines [183] as well as its role as a semi-permeable membrane that restricts the movement of metabolites and drugs from entering circulation [184]. A comprehensive review of the current gut-on-chip devices was recently published by Bein et al [185] and a summary of recent advances in 3D bioprinting the gut is also available from Wengerter et al [186]. This section will highlight recent works in the field as well as potential opportunities for integrating fluidics and 3D bioprinting for novel gut-on-chip systems.
Early research in microfluidic models of the human gut focused on emulating the permeability of the intestine to study drug absorption. A 2009 study aimed to create a microfluidic device to evaluate intestinal absorption of nutrients and compounds [187]. The device incorporated Caco-2 cells seeded on type I collagen coated on a membrane with 1 µm pores. The intestinal model was able to successfully recreate the permeability of cyclophosphamide (an anti-cancer drug) and Lucifer yellow (a high molecular weight fluorescent compound). Since this study, the models have become more advanced to incorporate more physiologically relevant morphology and better analytical methods. Gao et al performed a similar study with curcumin permeation through the epithelial barrier [188]. To characterize the concentration of the drug that had been absorbed, the effluent was run through a mass spectrometer; this automated system was able to quickly and accurately quantify the permeability of the compound and validate the performance of the epithelial barrier. A model containing the villus microstructures characteristic of the intestines was developed by Pocock et al [189]. This study focused on the absorption of lipophilic products of SN38 (an oral chemotherapeutic). The device was able to demonstrate better biological relevance compared to a Transwell model due to the presence of fluid flow inducing 3D morphology of the Caco-2 cells. Though many devices use semi-permeable membranes to separate flow channels and support cell culture, a 2017 study used a membrane-free method to study the permeability of the intestinal epithelium [184]. A commercially available, three-channel microfluidic platform (OrganoPlate) was loaded with a type I collagen gel to separate two fluid channels. One side of the gel was loaded with Caco-2 cells which formed a confluent epithelium expressing tight junctions and brush borders. Epithelial barrier leakage was studied by 4.4 kDa TRITC-dextran and 150 kDa FITC-dextran. Staurosporine and aspirin were delivered to the cells to study the impact of the drugs on the permeability of the membrane; the system was able to demonstrate that staurosporine concentrations as low as 0.36 µM and aspirin concentrations of 40 mM were able to cause significant damage to the epithelial barrier. High-throughput systems like this one have the potential to be used in large scale commercial applications for drug development and screening.
More recent work has aimed to recreate the villus structures of the luminal epithelium. A 2013 study by Kim et al demonstrated the ability to induce the morphogenesis of Caco-2 cells into villus structures by stimulating luminal flow [190]. Not only were the microstructures formed, but the epithelial cells were able to differentiate into absorptive, mucus-secretory, enteroendocrine, and Paneth cell types which yielded a more representative model of the intestine. The differentiated 3D intestinal structure showed increased drug metabolism compared to stationary, monolayered Caco-2 cells. Another study adapted a commercially available well insert (EpiIntestinal™ [Mattek]) into a multi-organ device to study sustained co-culture and inter-organ communication [191]. The insert contains primary human small intestinal ECs that are polarized and differentiated to yield a representative biological response. A different method of recreating the villus structures was published by Shim et al [192]. Instead of inducing morphological changes with flow or mechanical deformation, Caco-2 cells were seeded on a microfabricated collagen scaffold with finger-like protrusions. Observation after hematoxylin and eosin (H&E) staining confirmed more physiologically relevant morphology and enzyme activity compared to the cells cultured on a flat substrate. Though traditional gut-on-chip systems use Caco-2 cells due to their accessibility and facile handling, the intestinal carcinoma cell line may have limitations preventing accurate biological behavior [193]. To circumvent these limitations, a 2018 study incorporated primary human small intestine cells derived from the duodenum [194]. Crypt structures were isolated from biopsy-derived organoids and were transferred into the device in which they formed a coherent layer of cells over 8 to 12 days. Polarization and differentiation of cells was observed as the villus morphology was maintained throughout the experiment. This study makes significant progress towards the development of organ-on-chip systems derived from primary cells which shows promise for the use of these systems for personalized medicine in which treatments can be tested prior to administration to the patient. Finally, a unique approach to maintaining physiological properties ex vivo was developed by Yissachar et al [195]. Unlike other models that incorporate cells into the device, this device incorporated a section of intestine obtained from perinatal mice. Incorporating the entire segment of tissue maintained the microstructures found in vivo as well as the composition of a variety of cells including immune and nerve cells. This model facilitated the study of the complex interactions between the unpurified cells of the nervous system, immune system and gut through its incorporation of complex native tissue with cellular diversity and undisturbed physiological structure.
While the intestinal epithelium alone is limited in its value as a culture model, integrated systems including other organs and the microbiome show the broader impacts of the gut on the rest of the body. Several studies have integrated the gut with the liver to study drug toxicity and metabolism. One model of the gut-liver relationship focused on the ability of the intestinal barrier to reduce liver exposure to nanoparticles [196], while another has observed variable response to epirubicin, irinotecan, and cyclophosphamide in a lung-liver-gut microfluidic system [197]. Chen et al developed a liver-gut system that demonstrated that the gut can genetically regulate the production of bile acid by the liver and the two organs further communicate under inflammatory conditions to yield an augmented inflammatory response [198]. Another model integrating the gut and kidney demonstrated variable viability of renal cells as a result of absorbed digoxin, colestyramine, and verapamil [199]. Connected organ systems like these show promise for early prediction of toxicity prior to drug trials in animals and humans. Recent studies implicating the gut microbiome in digestion and disease have led to the development of models incorporating bacteria [183]. Some models demonstrate the role of the microbiome on membrane permeability [200] and others observe variable gene expression under various co-culture conditions depending on the environmental conditions such as oxygen and presence of other bacterial species [201]. One challenge of incorporating the human gut microbiome in an organ-on-chip system is recreating the anaerobic conditions that exist in the colon. These conditions are essential to supporting the diversity of species found in the gut and play a significant role in shaping the behavior and metabolism of the bacteria and the host [202]. Two obligate anaerobes were successfully sustained in co-culture with a gut-on-chip system using an anoxic-oxic interface [202]. Devices that can recreate the physiological microenvironment of the gut are essential in the development of microfluidic models that demonstrate the biological behavior of the microbiome in the body.
Gut-on-chip models can also be used to study diseased tissues. Irritable bowel disease (IBD), ulcerative colitis (UC), and Crohn's disease are all diseases of the gastrointestinal tract that are characterized by inflammation in the bowels [203]. A 2016 study used an organ-on-chip system to study the role of bacteria in repairing the inflamed gut epithelium [204]. Eight probiotic strains of bacteria were introduced to the device to remedy inflammation caused by pathogenic E. coli. Models like this may be integral in the development of new therapies for chronic inflammatory diseases without the use of traditional pharmaceuticals.
While extensive effort has been devoted to the development of gut-on-chip systems, relatively little work has focused on 3D bioprinting the tissue; a review by Wengerter et al discusses the current 3D bioprinting methods used in engineering gut tissue [186]. Prior to the use of bioprinting, 3D-printed plaster molds had been used to create porous poly(Lactide-co-glycolide) (PLGA) scaffolds for the seeding of intestinal ECs [205]. Since then, 3D bioprinting has been used to print multi-layer gut tissue. Madden et al printed a bilayer construct composed of human intestinal myofibroblasts (interstitium) and human intestinal enterocytes (epithelium) in Novogel® (Organovo, Inc.) (figure 8) [206]. The structure was printed into a cell culture well insert in the absence of flow. The epithelium did not form the villus microstructures. However, differentiation into Paneth, enteroendocrine, and goblet cells was observed, and the epithelium showed the expression of villin. The incorporation of both the interstitium and the epithelium provided a more comprehensive model for drug absorption compared to the microfluidic models only composed of a monolayer of Caco-2 cells. Kim et al demonstrated the use of 3D bioprinting to create an array of 250 µm villus structures made with a 4% collagen gel seeded with Caco-2 cells [207]. The Caco-2 cells had over 90% viability and showed expression of MUC17, E-cadherin, and alkaline phosphatase—all indicators of differentiation within the epithelium. Additionally, the differentiation markers were expressed earlier than the control sample in which cells were seeded on top of the same structure. One technology with promise for the development of new models of the intestines is coaxial 3D bioprinting. Coaxial printing can generate hollow fibers and has been applied to the creation of vasculature for nutrient delivery [208, 209], but may also be adapted for the creation of 3D gut models. While organ-on-chip technology and 3D bioprinting have not yet been integrated for the creation of gut models, there are opportunities to integrate the two technologies to improve uniformity and create complex barriers to better represent the physiological role of the gut in the human body. Progress in organ-on-chip and bioprinted models of gut is summarized in table 8.
Figure 8. The use of microfluidics and 3D bioprinting to create ex vivo gut models. (A) An organ-on-chip device recreating the intestinal epithelium interface with vasculature. (i) A schematic of the fluidic device showing the upper (epithelial; blue) and lower (microvascular; pink) cell culture microchannels separated by a porous, ECM-coated, PDMS membrane. Adjacent vacuum chambers can be used to mechanically stimulate the tissue barrier. (ii) Illustrated fabrication protocol to produce the intestine-on-chip model from human-derived cells. Reproduced from [194]. CC BY 4.0. (B) 3D bioprinting a bilayer model of the gut interstitium and epithelium. Like their in vivo counterparts, the apical cells in the tissue model express common biomarkers of the gut epithelium. Reprinted from [206]. Copyright 2018, with permission from Elsevier. This article was published in iScience, volume 2, Madden et al [206], Copyright Elsevier (2018).
Download figure:
Standard image High-resolution imageTable 8. Summary of progress related to gut models.
| Reference | Description | Major advance |
|---|---|---|
| Gut-on-chip (without bioprinting) | ||
| Imura et al [187] | Cultured gut endothelial cells (ECs) on a microporous membrane coated with collagen type I | The system matched the intestinal permeability for cyclophosphamide and Lucifer yellow |
| Gao et al [188] | Seeded gut ECs on a polycarbonate membrane | Directly coupled a mass spectrometer to the device to characterize the effluent |
| Pocock et al [189] | Gut ECs spontaneously formed villi-like structures when cultured on polycarbonate in the presence of fluid flow | Observed partitioning of a lipophilic chemotherapeutic drug, noticed 3D tissue morphology in the presence of fluid flow |
| Trietsch et al [184] | Loaded type I collagen gel into the device and seeded gut ECs on one side | Observed the negative impact of drugs on membrane permeability without the use of a semi-permeable membrane |
| Kim et al [190] | Coated a porous PDMS membrane with type I collagen and Matrigel and seeded gut ECs | Observed spontaneous villi formation in the presence of flow as well as cell differentiation into mucus-secreting, absorptive, enteroendocrine, and Paneth cells |
| Maschmeyer et al [191] | Adapted the EpiIntestinal™ well for culturing polarized, differentiated, small intestinal ECs | Used a fluidic circuit to connect effluent to other skin, liver, and kidney-on-chip models |
| Shim et al [192] | Micropatterned collagen to have finger-like protrusions and seeded gut ECs on top to form villi without other stimulus | Microfabricated a collagen scaffold instead of using self-assembly to create villi |
| Kasendra et al [194] | Seeded patient-derived duodenum epithelial organoids on an extracellular matrix (ECM)-coated membrane in a device with fluid flow and mechanical stimulation | Replaces the carcinoma gut EC line with primary human cells |
| Yissachar et al [195] | Placed sections of intestine derived from perinatal mice to preserve native microstructure | The in vitro model preserved in vivo morphology and cell diversity to study immune involvement |
| Kim et al [200] | Gut epithelium is seeded on an ECM-coated PDMS membrane, the tissue is exposed to fluid shear stress and cyclic strain. Lactobacillus Rhamnosus was also cultured on the luminal side of the gut epithelium | Incorporated normal gut bacteria into an in vitro model without compromising host cell viability |
| Shah et al [201] | Developed a two-tiered culture system in which gut ECs are seeded on a collagen-coated microporous membrane and Lactobacillus rhamnosus are seeded on a mucin-coated nanoporous membrane | Facilitates indirect communication between the host and microbes in the system |
| Shin et al [202] | Created a longitudinal oxygen gradient within the microfluidic device to create a relevant microphysiological environment for gut ECs seeded on the collagen-Matrigel-coated membrane with two obligate anaerobic bacteria | Generated a stable oxygen gradient capable of sustaining obligate anaerobic microbiota with host cells |
| Kim et al [204] | Pathogenic E. coli in tandem with a halt in peristalsis-like motion induced inflammation in a standard gut-on-chip device, eight probiotic bacteria strains were delivered to remedy inflammation | Observed overgrowth of pathogenic bacteria and a therapeutic effect of probiotic bacteria in vitro |
| Bioprinted gut | ||
| Madden et al [206] | A bilayer intestinal model composed of myofibroblasts and ECs was printed used Novogel® | Cell differentiation was observed in the absence of shear stress over the surface |
| Kim et al [207] | Printed a collagen bioink seeded with ECs to create an array of 250 µm villus structures | Observed >90% viability and expression of differentiation markers of epithelial lineages |
Kidney
The kidney is responsible for acting as a filter for blood, removing waste and reabsorbing useful substances, such as water and glucose. The kidneys play an important role in drug excretion and nephrotoxicity is of importance in drug development and testing. Kidney-on-chip models mimic key features of the human kidney, including structural, mechanical, transport, absorptive and physiological properties [210, 211]. One factor limiting the development of improved in vitro kidney models is the kidney's cellular and structural complexity [212]. A contributing factor to this complexity is the kidney's functional unit, the nephron, which is composed of more than 13 different EC types, surrounded by a supporting array of vascular, stromal, and immune cells [212].
Two of the earliest published kidney-on-chip papers share a two-compartment design [213, 214]. A study by Jang et al featured a simple multi-layer device formed by constructing two compartments, one flow and one static chamber, separated by a porous membrane [213]. This system used rat inner medullary collecting duct cells, exposed to laminar shear stress in the channel of approximately 1 dyn cm−2 for 5 h. A second report, by the same group, utilized a similar setup, but with human proximal tubular epithelial cells [214]. The proximal tubular cells were exposed to lower stress of approximately 0.2 dyn cm−2. Other microfluidic kidney-on-chip platforms approach the challenge of mimicking the kidney's complexity by modeling one portion of the kidney. Recent work includes glomerulus on-chip [215, 216], proximal tube-on-chip [214, 217], and distal tubule-on-chip [218].
The human kidney glomerulus acts as the major site for blood filtration [215]. An in vitro model of the glomerulus could serve as a tool for drug discovery and improved understanding of kidney-disease mechanisms. Previously, fabrication of a kidney-glomerulus-on-chip was not possible due to the lack of functional human podocytes. Work by Musah et al in 2017 overcame this challenge via an efficient method for directing human iPSC differentiation into podocytes [215]. The differentiated podocytes were then co-cultured with human glomerular endothelial cells in a microfluidic device, recapitulating the glomerular tissue interface. While this study did not rebuild an entire glomerulus, it reconstituted critical functions of the human glomerulus well, which has not been previously modelled. Another example of glomerulus-on-chip was developed by Zhou et al to develop a model of hypertensive glomerulopathy. Glomerular hypertension results in glomerular sclerosis, which then exacerbates end-stage renal disease [216]. To better understand the mechanism of glomerular sclerosis, a glomerulus-on-chip was developed. This glomerulus-on-chip model was comprised of two channels, lined by closely opposed layers of glomerular endothelial and podocyte cells. The results showed that glomerular mechanical forces had a crucial role in cytoskeletal rearrangement and damage to cells leading to the glomerular leakage. These findings demonstrated that the glomerulus-on-chip could provide a platform for both drug and toxicity screening, as well as a personalized platform for glomerular disease.
The proximal tubule in the human kidney is the primary site in the nephron for waste excretion. As a result, it is a primary target for drug-induced nephrotoxicity [219]. In 2016, Weber et al developed a 3D flow-directed human kidney proximal tubule MPS [219]. The described system replicated the proximal tubule's polarity, marker proteins, biochemical activity, and the secretory and resorptive processes of the native proximal tubule, serving as an ideal platform for ex vivo modeling of drug clearance and nephrotoxicity. Other models of the kidney proximal tubule mimicked the apical shear stress experienced in living proximal tubules [214] and a lab-on-chip model was used for testing under flow conditions [217]. The first model, as previously described in our review, recapitulated the apical shear stresses in vivo and more closely mimicked in vivo responses than cells in conventional culture conditions [214]. This is one of the earliest toxicity studies using primary human kidney proximal tubular epithelial cells in organ-on-chip systems and has applications as a useful tool for evaluating renal toxicity. Extensions of this work utilized a hollow fiber membrane structure with renal proximal tubule epithelial cells (PTECs) and then embedded this structure in a lab-on-chip bioreactor system to mimic flow conditions [217]. The incorporation of microenvironmental characteristics such as cell pattern, 3D structure, and fluid flow can lead to improved renal substitution technology and drug testing platforms [214, 217].
Unlike the glomerulus and proximal tubule, few studies have explored the distal tubule-on-chip. However, despite the scarcity of studies, the distal tubule segment of the nephron remains a relevant structure for clinical practice due to its role in sodium, potassium, and cation homeostasis [220]. A study by Baudoin et al developed a microchip with distal tubular cells cultured in microchannels [218]. The results showed that the number of viable Madin Darby canine kidney (MDCK) cells decreased under higher shear stress values, suggesting that MDCK cells may not be able to adapt to the flow rates observed in the proximal tubule. Integration of models of multiple structures within the kidney in combination to work towards a complete kidney-on-chip have also been shown. For example, Weinberg et al designed a device for replicating a single nephron, including the glomerulus, the proximal tubule, and the loop of Henle [221]. In order to improve these models, studies of less well-characterized regions of the kidney will be needed [222].
An alternative approach to modeling the kidney is the generation of organoids. Organoids are miniature, 3D diverse cell aggregates, often derived from stem cells [223]. Organoids demonstrate great promise for in vitro studies of human diseases but are often avascular in static culture. The incorporation of vasculature and perfusion into kidney organoids has been shown to induce morphological maturation, opening new avenues of applications for organoids [224]. More recently, 3D bioprinting has emerged as a technology for use in building organoids [225]. Recent advances in drug screening for nephrotoxicity and in disease modeling are using bioprinting to create organoids [226]. Recently, Higgins et al used the NovoGen MMX 3D bioprinter to create kidney organoids [226]. This technique utilized automation allowing for improved reproducibility. This method also showed much higher efficiencies in generating kidney organoids from approximately 4000 cells, allowing for the generation of more organoids from the same number of cells. These organoids were tested for their response to doxorubicin to demonstrate their ability to be used as a predictive model for drug toxicity.
The combination of 3D bioprinting with microfluidic technologies is necessary for the development of physiologically simulating, complex, and long-term viable micro-organ arrays [227]. Recent work from the Lewis lab presented a bioprinting technique for creating 3D human renal proximal tubules housed in perfusable tissue chips (figure 9) [228]. The developed 3D proximal tubules-on-chip demonstrated enhanced epithelial morphology and functional properties, compared to the 3D controls (both with and without perfusion). This 3D chip model can be used for elucidation of drug induced renal damage mechanisms. This method opens new avenues for fabrication of 3D bioprinted kidney tissues-on-chip, which could facilitate advances in regenerative medicine and drug development. Progress in organ-on-chip and bioprinted models of kidney is summarized in table 9.
Figure 9. Bioprinted proximal tubule of the kidney on a perfusable chip. (A) Anatomical illustration of a nephron highlighting the convoluted proximal tubule, (B) Illustrated protocol for the printing of sacrificial Pluronic gel to form a hollow lumen in a gelatin-fibrinogen hydrogel. Proximal tubule epithelial cells (PTECs) were seeded in the vacated lumen. (C) Photographs of the manufacturing process illustrated in B. (D) Confocal microscopy image of the printed tubule. Actin is stained in red and nuclei are blue, the white dotted line denotes the tissue cross-section shown in the white box below in which PTEC cells circumscribe open lumens. Scale bar = 500 µm. (E) Higher magnification view of the wall of PTECs formed in the open lumen. Scale bar = 200 µm. (F) Confocal image of perfused proximal tubule-on-chip, Na/K ATPase is stained in red, acetylated tubulin is orange, and nuclei are blue. Scale bar = 50 µm. Reproduced from [228]. CC BY 4.0.
Download figure:
Standard image High-resolution imageTable 9. Summary of progress related to kidneys.
| Reference | Description | Major Advance |
|---|---|---|
| Kidney-on-chip (without bioprinting) | ||
| Jang et al [219] | Rat inner medullary collecting duct cells cultured inside of a PDMS channel with a fluidic shear stress of 1 dyn cm−2 applied for 5 h to generate an in vivo tubular-like environment | Enhanced cell polarization and cytoskeletal reorganization suggest recapitulation of the renal tubule system |
| Jang et al [220] | Microfluidic device lined by human kidney ECs split into two adjacent channels by a porous membrane with kidney ECs cultured on top exposed to apical fluid shear stress (0.2 dyn cm−2) | Exposure to shear stress mimicking that of kidney tubules results in enhanced epithelial polarization and primary cilia formation |
| Musah et al [215] | Co-cultured hiPSC-derived podocytes with human kidney glomerular endothelium in a fluidic device | Produced glomerular basement-membrane collagen and recapitulated the tissue interface of the glomerulus |
| Zhou et al [216] | Two channels lined by glomerular endothelial cells and podocytes experiencing physiological flow conditions | Revealed that glomerular mechanical forces play a role in cell cytoskeletal rearrangement and damage to the cells leads to glomerular leakage seen in hypertensive nephropathy |
| Baudoin et al [218] | MDCK cells cultured inside of a PDMS microchip with varied flow rates | Reduction in cell growth rate after exposure to ammonium chloride demonstrated potential for use in chronic toxicity studies |
| Weber et al [219] | Primary proximal tubular epithelial cells seeded in a cylindrical microphysiological system | Under flow, PTECs self-assemble to form a 3D tubular structure and polarize, mimicking the human proximal tubule |
| Homan et al [224] | Cultured vascularized kidney organoids under flow on fluidic chips | Enhanced cell polarity and mature gene expression in podocyte and tubular cell compartments than in static culture |
| Bioprinted kidney | ||
| Higgins et al [226] | Used NovoGen MMX 3D bioprinter to generate kidney organoids from 4000 cells | Demonstrated ability to print kidney organoids in a 96 well plate for use in high-throughput drug screening |
| Kidney-on-chip (with bioprinting) | ||
| Homan et al [228] | Bioprinted 3D renal proximal tubules and housed them in a perfusable chip | Epithelial barrier responds to Cyclosporine A, a nephrotoxin, in a dose-respondent manner |
Cancer
While organ-on-chip systems can be used to recreate healthy tissue, extensive effort has also been devoted to recreating diseases within microfluidic devices [15, 17]. Perhaps the most well studied disease with organ-on-chip technology is cancer. Cancer-on-chip systems are designed for three main purposes: to understand the role of the tumor microenvironment in cancer progression, to study the biological processes associated with metastasis, and examine the efficacy of drug therapies. While the governing phenomena of these three areas vary greatly, the use of microfluidic devices allows customized geometries that facilitate the study of specific biological and environmental factors in each domain. Most microfluidic devices study recreated 2D tissue interfaces, which allow optimal observation of artificially created gradients and cell mobility. Though they are easy to observe, 2D models do not incorporate the necessary 3D architecture and cellular interactions that are prerequisites for demonstration of physiologically relevant behavior [229, 230]. The tumor spheroid has been the most widely adopted 3D construct for studying each factor as they are easy to observe, provide quantitative data, can be composed of several cell types, produce their own ECM, and have the ability to exhibit metastatic events [229]. These spheroids have been studied in a variety of environments ranging from well plates to microfluidic devices. This section will discuss the current microfluidic and bioprinted models of cancer and the potential for devices that incorporate state-of-the-art manufacturing with engineered microdevices.
The application of cancer-on-chip models for drug screening may yield excellent tools for the development of high-throughput assays used to identify compounds as potential drugs or to evaluate the toxicity and efficacy of existing pharmaceuticals [231]. While current industrial practice primarily uses 2D culture systems and research is being conducted on well-based spheroid assays [232], microfluidics has the potential to revolutionize drug screening. Lab-on-chip platforms such as the system developed by Yu et al show potential for creating 3D cell aggregates for drug screening [233]. This device both creates the aggregates and organizes them into an easily observable array of breast cancer models for pharmaceutical testing. Another device created by Fan et al formed and analyzed glioblastoma multiform (GBM) spheroids in a poly(ethylene) glycol diacrylate (PEGDA) chip [234]. This system facilitated the study of concurrent application of two common anti-GBM drugs, pitavastatin and irinotecan, and observed cell detachment and death on the surface of the spheroids. While microfluidic devices created by traditional manufacturing methods are sufficient for academic studies, inkjet or extrusion 3D bioprinting techniques may be used to further control, optimize, and scale the manufacturing process. While there are several challenges in the development of screening methods and devices that are suitable for commercial use, 3D bioprinting can make microfluidic designs like these feasible for commercial scale use as the devices become the next generation tools of drug development.
Cancer metastasis is another important process that has been isolated to microfluidic platforms. Metastasis describes the event of cancer cell migration from the primary tumor to another part of the body and is responsible for most cancer-associated deaths [235]. In cases in which the tumor metastasizes to a distant region of the body, the process involves invasion of the blood vessels (intravasation), circulation in the blood, and invasion of the surrounding tissue through a vascular wall (extravasation) [236]. Many cancer-on-chip systems have already been developed to study the various aspects of this process and have been reviewed by Caballero et al in 2017 [236] and Sleeboom et al in 2018 [237].
Both intravasation and extravasation involve invasive cancer cells penetrating blood vessels. One intravasation model developed by Zervantonakis et al observed fibrosarcoma cells invade a vessel lined with ECs [238]. The device was composed of two cell culture channels separated by a collagen matrix. The invasive fibrosarcoma cells entered the matrix and aggregated near the endothelium prior to entering the simulated vessel. Similar models have been used to study the invasive behavior of glioblastoma by observing EC-driven invasive behavior of glioma initiating cells through a collagen matrix [239] and to elucidate the relationship between cancer cell density and migration towards vasculature [240]. Extravasation models demonstrate the opposite metastasis event in which the cancer cells begin within the walls of the EC-lined vasculature and invade into the surrounding tissue. Some studies focus on the extravasation process, studying the role of the endothelium. Others, however, study the role of the microenvironment in which the cancer is extravasating. Models created by Jeon et al and Zhang et al observe cancer penetration into artificial ECM. The device created by Jeon observed the invasive behavior of breast cancer [241], while Zhang et al studied the invasion of salivary gland carcinoma into a chemokine-loaded ECM [242]. A co-culture device created by Du et al demonstrated the relationship between cancer cell density and migration towards vasculature [240]. More complex models aimed to recreate the microenvironment of a common metastasis site, bone. Both Bersini et al and Marturano-Kruik et al studied the migration of breast cancer cells into the bone microenvironment on a microfluidic device [97, 243]. A recent two-channel device made by Jeon et al allows for the study of extravasation as well as the modification of the extravasation site by inputting chemical factors and other cell types [97]. While the chip was initially used to study the extravasation of breast cancer cells into a bone-mimicking microenvironment, the same design can be used to recreate other metastatic sites throughout the body with ease. Another unique device, designed by Xu et al in 2016, contains both intravasation and extravasation on a single microfluidic model [244]. Additionally, this model contained organoids representing the brain, bone, and liver on one, interconnected device. Complex models like this one are ideal for manufacturing via bioprinting due to the diverse organ structures and compositions required for proper function.
The tumor microenvironment is a complex set of chemical, biological, and mechanical cues from the surrounding cells, ECM, and fluid flow [245]. Traditional 2D cell culture models fail to recreate one, if any, of these important environmental factors. However, 3D microfluidic models have been used to study each cue's impact on the tumor. A comprehensive review of microfluidic chips replicating various aspects of the tumor microenvironment was published in 2017 [245]. This review highlights systems that may benefit from 3D bioprinting.
Fluid force plays an important role in influencing cellular migratory behavior [246]. These fluid flows have been shown to have an impact on angiogenesis and metastasis of tumors. Existing models include cell-laden tubular structures developed to study the role of shear stress on the development of new blood vessels [247] to devices with hydrogel barriers used to simulate interstitial flow over a malignant growth [248]. Microfluidics are an essential tool in studying fluid-based mechanical stresses as these devices allow fine control over fluid velocity and shear. Facile manipulation of the fluidic microenvironment facilitates better simulation of physiological conditions ex vivo.
Perhaps the most studied phenomena using microfluidic cancer models is the effect of neighboring cells. Three major types of cells have been studied with a wide array of cancer types. Macrophages, cells associated with immune response, have been studied for their role in tumor and immune suppression. Two separate studies demonstrated the ability of macrophages to infiltrate adenocarcinoma [249] and breast cancer [250] cell aggregates in adjacent channels. Each of these devices feature multiple compartments with parallel fluid flow to supply nutrients. Another microfluidic device was used to observe the ability of transitional cell carcinoma of the bladder to reprogram macrophages [251]. This study yielded interesting results pertaining to the impact of secreted signaling molecules (lactate and quercetin) on each cell type to cell type.
Beyond macrophages, extensive work has also been done on the role of fibroblasts, which are cells that are responsible for the creation and maintenance of the ECM. The primary goal of some devices is to sustain a co-culture with tumor cells to be used for studying cell-specific drug response [252], while many aim to observe fibroblast-induced invasive behavior of the tumor. In each of the invasion models, migration of cancer cells was observed across a hydrogel matrix. Bischel et al developed a 3D model through a fluid-based manufacturing method to recreate breast cancer in vitro [253]. This model is notable as its 3D structure facilitated the inclusion of important biological structures, such as the basement membrane, which were left out of similar 2D models. 3D bioprinting may be useful in enhancing these models to create relevant cancer architectures with cellular heterogeneity to yield more information about the role of fibroblasts in invasive behavior from tumors rather than unorganized cells.
Biochemical gradients are another aspect of the tumor microenvironment that has been studied using microfluidic devices. Most research on physiological gradients in the context of cancer focuses on the distribution and concentration of small molecules (growth factors and chemokines) and oxygen. These phenomena are of interest as altering naturally occurring gradients may lead to methods of isolating and treating disease. For example, breast carcinoma cells were studied in a gradient of epidermal growth factor (EGF) patterned by passive diffusion through a gel [254]. This study concluded that higher concentrations of EGF increased invasive behavior and suggested that it may be used as a drug screening platform. While some gradients were formed by passive diffusion throughout a gel, others were created and sustained by biological and chemical reactions. A chemokine (signaling proteins that induce cell migration) gradient was formed across a hydrogel by chemokine-expressing cells in one channel and chemokine-sequestering cells in another [255]. The migration of breast cancer cells towards the chemokine-producing cells was observed.
Oxygen gradients are another interesting component of the tumor microenvironment; especially as hypoxic conditions are known to lead to aggressive and resistant tumor behavior [256]. Several microfluidic devices have been created using variable gas supplies and controlled chemical reactions to develop concentration gradients. The devices using gas diffusion to create gradients exposed one channel to a high oxygen concentration and another to nitrogen gas (devoid of oxygen) [257, 258]. These systems achieved gradients ranging from 0% to 20%. Chen et al developed a system with an oxygen producing and oxygen scavenging reaction; this system produced a non-linear gradient across PDMS ranging from 0% to 11% [259]. A similar system, dependent only on an oxygen scavenging reaction and passive gas diffusion from air, maintained a gradient from 2% to 11% [260]. Another system was designed by Wang et al to study dual gradients of chemical signals and oxygen [261]. This project continued to study the effect of drugs on cancer cells within hydrogel matrices to understand drug metabolism in physiologically relevant conditions. A recent paper demonstrated the creation of an oxygen gradient (5 mg l−1 to 40 mg l−1) over a 2D cell culture channel by controlling media oxygenation [262]. This strategy allows for better control of the gradient by incorporating continuous sensing and facile modulation. In general, these types of microfluidic devices have been used to study the activity of hypoxia-dependent anti-cancer drugs like tirapazamine (TPZ) and may be useful in providing pre-clinical data on other oxygen sensitive therapies for cancer. Each of the models using TPZ confirmed the activity of the drug in hypoxic conditions by observing cell death exclusively in the poorly oxygenated regions of the devices.
The final component of the tumor microenvironment is the ECM. The ECM is the network of proteins around a cell that provide the structure for cell adhesion and growth. ECM properties such as stiffness and orientation impact the adhesion and progression of cancer [263]. While the study of ECM-dependent cell behavior does not require microfluidic devices, some have been created to study the remodeling of the ECM due to cancer cells. While the device structures are not unique compared to those used to study other phenomena, systems like those created by Gioiella et al are designed to observe modified collagen, hyaluronic acid, and fibronectin structures [264]. Bioprinting methods using multiple materials, as demonstrated by Weiss et al, provide a method of better controlling the ECM by introducing spatial and chemical variation [265].
Many 3D printing techniques have also contributed extensively to cancer research. In-depth reviews discussing the relationship between bioprinting and cancer research have been published by Knowlton et al [266] and Zhang et al [41]. Studies that modify the geometry of the cancer microenvironment have observed significant changes in the morphology and migration of cancer cells [267, 268]. In addition to the geometry of scaffolds, bioprinting with multiple materials can yield constructs with unparalleled spatial variation. For example, Weiss et al demonstrated the use of a four-head inkjet bioprinter that could vary the integration of fibrinogen, thrombin, and two growth factors into a 3D scaffold [265]. This printing method allowed the group to control not only the density and mechanical properties of the scaffold, but also the concentration of growth factors within the material. In addition to scaffold creation, bioprinting techniques can assist in the formation of 3D cell constructs. A similar printing method was also used for the controlled deposition of ovarian cancer cells and fibroblasts [269]. Angled printheads allowed simultaneous deposition of nanoliter quantities of cell media in an efficient and consistent process, making it an ideal technique for commercial devices requiring high precision and uniformity. In addition, 3D printing has also been used to create a microwell array for the formation of breast cancer spheroids and iPSC-based embryoid bodies [270]. Similar work has also been performed with breast cancer spheroids by printing arrays of cell-laden hydrogels in a PEGDA device [271]. Techniques like these yield precise control over the size of cell aggregate, leading to optimized constructs for physiological modeling. A unique method of forming heterogeneous self-assembled breast cancer spheroids was published by Jiang et al in 2017 [272]. This method bioprinted alginate and gelatin in a unique pattern with fibroblasts on the periphery and breast cancer cells in the center. Over 30 days, the fibroblasts, activated by the breast cancer cells, migrated toward the center and formed heterogenous 3D breast cancer/fibroblast spheroids. Though slower than the other manufacturing techniques, this method created self-assembled, heterogeneous tumor models by simply supplying the proper environment. With the potential to create relevant 3D cell structures and the scaffolds to support them, bioprinting has demonstrated its potential to improve existing cancer models; integration with microfluidics shows potential to create powerful devices for studying disease and developing therapies in the future.
Several publications have outlined manufacturing systems that combined 3D bioprinting and microfluidics to study cancer cells. Hamid et al created two platforms for the creation of uniform, cell-laden microfluidic devices that study breast and liver cancer. Both platforms boasted the ability to deposit and cure PDMS into the desired design, perform local plasma treatment on the device, and deposit cell-laden bioinks into the device; the platforms only differed by the use of photolithography [273] or extrusion-based printing (figure 10) [274]. Though cancer cells were successfully printed into the microfluidic device and remained viable after manufacture, the devices have only been used to observe the metabolism of 7-ethoxy-4-trifluoromethyl coumarin (EFC) by breast cancer cells. Another platform integrated single-cell printing capability with a scalable microfluidic device. The system, developed by Mi et al had the ability to pattern cells with a precision of 10 µm by depositing volumes as small as 0.1 nl [275]. This system was used to verify the inhibition of migration by the anti-cancer drug paclitaxel on human breast cancer cells. This system demonstrates the potential of bioprinted-microfluidic systems to be adapted for commercial use; it not only offers high speed and precision, but also the scalability that is required for industrial use. Future studies using similar printing-based manufacturing platforms should aim to recreate the tumor microenvironment, reproduce metastatic conditions, and develop scalable drug screening devices to build further insight into the mechanisms of cancer and novel therapeutic strategies for fighting the disease.
Figure 10. Integrated 3D printing technology for the production of a microfluidic device to study co-cultured breast and liver cancer. (A) (i) Photograph of the multi-nozzle bioprinter (ii) focusing on the modular print heads able to extrude PDMS and bioinks as well as UV crosslink and plasma treat the printed construct. (B) SEM image of the PDMS microfluidic device showing the high-resolution channel production with excellent precision and uniformity in both the (i) linear regions and (ii) corners. (C) (i) SEM image of distributed liver (HepG2) and breast (MDA-MB-231) cancer cells in the device channels. (ii) Magnified view of cells in the channels. (iii) Individual breast cancer and (iv) liver cancer cells. Reproduced from [274]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageIn summary, the synergistic combination of bioprinting and microfluidics has the potential to improve each type of tumor model. Tumor microenvironment and metastasis models can benefit from the ability to mimic complex cell patterning within and surrounding tumors. Some 3D printing methods may offer simple solutions to printing constructs with biochemical gradients as well. Other techniques like coaxial bioprinting may also be used to replicate vasculature and other vessels to study and understand metastasis. Finally, bioprinting may be the solution to creating scalable microfluidic devices for drug screening; the technology provides an efficient method of mass-manufacturing uniform devices composed of inorganic and biological materials in a one-step process. The next generation of complex, commercializable microfluidic devices for cancer research may be achievable with the use of 3D bioprinting. Progress in organ-on-chip and bioprinted models of cancer is summarized in table 10.
Table 10. Summary of progress related to cancer.
| Reference | Description | Major advance |
|---|---|---|
| Cancer-on-chip (without bioprinting) | ||
| Yu et al [233] | Created and organized alginate-encapsulated breast cancer spheroids for drug screening | Used to observe dose-dependent response to doxorubicin |
| Fan et al [234] | Created and organized glioblastoma spheroids for drug screening | Observed drug response localized to spheroid surface cells |
| Zervantonkis et al [233] | Simulated intravasation through replication of vasculature and seeding of fibrosarcoma | Demonstrated that intravasation was modulated by the presence of TNF-alpha |
| Jeon et al [241] | Simulated extravasation through endothelial cell barrier into a collagen matrix | Device enabled quantification of extravasation behavior of breast cancer cells |
| Zhang et al [242] | Simulated extravasation into a chemokine-loaded hydrogel | Demonstrated impact of CXCL12, AMD3100, and CXCR4 on invasion |
| Du et al [240] | Co-cultured breast cancer, healthy mammary endothelial cells (ECs), and human umbilical vein endothelial cells (HUVECs) to model invasion | Used to elucidate the role of IL-6 in invasive cancer behavior |
| Bersini et al [243] | Simulated adenocarcinoma metastasis into the bone microenvironment composed of collagen and MSC-derived osteocytes | Demonstrated significance of CXCL5 signaling in extravasation to bone |
| Marturano-Kruik et al [97] | Seeded bone marrow mesenchymal stem cells (MSCs) and endothelial cells into decellularized bone matrix in a microfluidic device | Showed self-assembly of vasculature and bone structures in dECM, studied cancer drug resistance in the presence of interstitial flow |
| Jeon et al [276] | Modeled metastatic seeding by recreating the bone microenvironment using endothelial cells, mesenchymal stem cells, and osteoblasts in a hydrogel | Observed that the conditions in the metastatic site impacted cell seeding in the gel |
| Xu et al [244] | Modeled metastasis in vitro by incorporating lung, bone, brain, and liver microenvironments on a single chip. Lung cancer cells were observed | Facilitated the preferential seeding of lung cancer cells to common metastatic locations |
| Bai et al [249] | Cultured lung adenocarcinoma aggregates in the presence of macrophages and HUVECS | Allowed study of the roles of different macrophages on cancer disaggregation |
| Huang et al [250] | Co-cultured breast cancer cells and macrophages in collagen and Matrigel | Demonstrated that invasive behavior was promoted by macrophage presences |
| Zhao et al [251] | Studied transitional cell carcinoma of the bladder's role in macrophage reprogramming | Observed in vitro reprogramming of macrophages to M2 phenotype due to cancer cells |
| Menon et al [252] | Gelatin-lined microfluidic channels supported co-culture of bone marrow stromal cells and hepatocarcinoma | Developed a simple, inexpensive co-culture device to study tumor-stroma interactions |
| Sung et al [277] | Passively loaded a microfluidic device with ductal carcinoma cells and mammary fibroblasts in collagen-Matrigel hydrogels | Observed in vitro collagen remodeling and invasive behavior due to cancer-fibroblast interactions |
| Liu et al [278] | Cultured patient-derived salivary gland adenoid cystic carcinoma cells and carcinoma-associated fibroblasts in Basement Membrane Extract | Carcinoma-associated fibroblasts triggered invasive behavior from cancer spheroids in a 3D gel |
| Li et al [279] | Induced salivary gland adenoid cystic carcinoma invasion through a Matrigel matrix using carcinoma-associated fibroblasts | Observed that CXCL12 and metallomatrix proteins promote invasive behavior, carcinoma cells migrate through tracks created by activated fibroblasts |
| Yu et al [280] | Three-chamber microfluidic device culturing non-small cell lung cancer and fibroblasts to observe local interactions and chemokine signaling | Identified GRP78 as a key genetic driver of invasive behavior. |
| Bischel et al [253] | Recreated mammary duct 3D structure by patterning healthy mammary ECs and DCIS cells encased in a basement membrane surrounded by fibroblasts | Demonstrates that fibroblasts play a large role in invasive behavior |
| Truong et al [254] | Generated an EGF gradient through a hydrogel to observe invasiveness | Invasive behavior is positively correlated to EGF concentration |
| Torisawa et al [255] | Used a tri-culture device with breast cancer and embryonic kidney cells to establish a chemotaxis assay for CXCL12 | Described the role of CXCL12 in cancer chemotaxis |
| Oppegard et al [257] | Created an oxygen gradient across an agarose gel seeded with breast cancer cells | Developed physiologically relevant in vitro oxygen gradients |
| Uchida et al [258] | Maintained an oxygen gradient along a 2D co-culture of liver cancer cells and HUVECs | Observed in vitro expression of hypoxia-related genes in endothelial cells |
| Chen et al [259] | Used confined chemical reactions in a microfluidic device to create a hypoxic microenvironment for lung cancer cells seeded on fibronectin | Observed cytotoxic effects of hypoxia and hypoxia-dependent drug activity |
| Wang et al [260] | Used a confined oxygen-consuming reaction to generate an oxygen gradient across lung cancer cells seeded on fibronectin | Cellular resistance induced by hypoxia induced resistance to cisplatin and efficacy of tirapazamine (TPZ) |
| Wang et al [261] | Created dual oxygen and chemical (drug) gradients on a single device for the 2D culture of human lung and cervical cancer | Integrated oxygen and chemical gradients in a single device |
| Orcheston-Findlay et al [262] | Controlled media oxygenation to develop an oxygen gradient across a 2D culture of Ishikawa cancer cells | Allows for facile modification of gradient concentration and composition by altering independent gas exchange processes |
| Gioiella et al [263] | Co-cultured perfused microtissues of fibroblasts and epithelial breast cancer cells | Demonstrated stroma invasion and extracellular matrix (ECM) remodeling by over expression of collagen and hyaluronic acid |
| Bioprinted cancer | ||
| Huang et al [267] | Used digital micromirror device (DMD)-based projection printing to create a poly(ethylene) glycol diacrylate (PEGDA) structure and seed cervical cancer cells | Implemented 3D printing to create a micropatterned structure to study cell migration |
| Zhu et al [268] | Adapted stereolithography-based 3D printing to create hydrogel scaffolds encasing hydroxyapatite nanoparticles to mimic the bone metastatic environment. Seeded breast cancer and MSCs to study aggregation and drug resistance | Used 3D printing to create a geometrically complex matrix structure to study bone metastasis |
| Hribar et al [270] | Implemented DMD printing to generate PEGDA microwells for the generation of breast cancer spheroids | Created a microwell platform using a biocompatible hydrogel for spheroid creation |
| Ling et al [271] | Used pressure-assisted value-based bioprinting to pattern gelatin droplets for the subsequent molding of PEGDA wells used for breast cancer spheroid culture | Use of 3D printing for the creation of tunable hemispherical microwells |
| Bioprinting | ||
| Xu et al [269] | Used an inkjet bioprinter to seed ovarian cancer cells and normal fibroblasts on a Matrigel substrate | Implemented inkjet bioprinting for controlled distribution of cancer cells |
| Jiang et al [272] | Used multi-nozzle extrusion bioprinting to facilitate self-assembly of heterogenous spheroids of breast cancer cells and fibroblasts in an alginate/gelatin matrix | Facilitated spheroid self-assembly resulting from cell migration through bioprinted constructs |
| Cancer-on-chip (with bioprinting) | ||
| Hamid et al [273] | Microfluidic device is printed with DMD technology and cells are seeded with extrusion bioprinting | 3D printing technology was used to fabricate every aspect of the device |
| Hamid et al [274] | A single printer with four printheads was used to print the PDMS enclosure and (breast and liver) cancer cell solutions within SU-8 internal architecture | Uses a maskless printing technology to fabricate all aspects of the microfluidic device |
| Mi et al [275] | Demonstrated a bioprinter able to deposit sub-nanoliter quantities of breast cancer cell suspension on a microfluidic device used to study cell migration | Demonstrates viability of bioprinting to position individual cells within microfluidic devices |
Challenges and future directions
Over the last few years, 3D bioprinting technology has shown great promise to mimic the structure and function of native tissues. A variety of biological tissues have been developed using 3D bioprinting and used for toxicity, biological, and disease modeling studies. Microfluidic systems have been an integral part of such bioprinted structures to ensure that the printed constructs will survive and function for a considerably long time. The discovery of iPSCs and their ability to differentiate into many cell lines have opened up new avenues to make personalized tissue constructs in a robust and reproducible way [281–284]. Such tissue constructs would be able to minimize the variability among cells from different people. There is an exciting opportunity for bioprinting to combine with genetic manipulation of cells using cell reprogramming in situ in an efficient manner [285]. Therefore, there would be a significant decrease in personalized tissue fabrication because traditional cell reprogramming in 2D systems requires sophisticated media and long culture period. There is growing evidence that reprogrammed cells in 3D environment can better support one another compared to traditional 2D cell cultures [15]. Moreover, 3D bioprinting can provide other biological and mechanical cues for cell survival and function in vitro. Such microenvironments would also be useful to study the fate of reprogrammed cells in in vivo-like conditions. In particular, bioprinted tissue-on-chip models would be able to determine safety and efficacy of drug candidates prior to pre-clinical and clinical testing. In this section we would like to highlight some challenges and future directions that might be useful for researchers in this emerging research area.
The synergistic combination of bioprinting and microfluidics has the potential to improve a wide array of physiological models. As the challenges previously associated with bioprinting, i.e. resolution, bioink materials, and the limited ability to co-print multiple cell types, are overcome by advances in bioprinting platforms, the integration of these two technologies has become more frequent, offering an exciting new avenue for improved preclinical models. Cell microenvironment and migration models can benefit from the ability to mimic complex cell patterning within and surrounding organs. Some printing methods may also offer simple solutions to printing constructs with biochemical gradients as well. Other techniques, like coaxial bioprinting, may also be used to better replicate vasculature and other vessels to study and understand complex organ systems. Finally, bioprinting may be the solution to creating scalable microfluidic devices for drug screening; the technology provides an efficient method of mass-manufacturing uniform devices composed of inorganic and biological materials in a one-step process. Advanced bioprinting with organ-on-chip platforms will also overcome the limitations and controversies of more traditional preclinical models. 3D printed tissues integrated with microfluidic systems have the potential to offer an alternative to animal studies, reducing the ethical issues associated with drug testing. While organ-on-chip platforms may use cells isolated from animals, these systems have the potential to perform hundreds of tests on a single chip, (as opposed to a single test on hundreds of animals), decreasing the quantity of animals sacrificed for research. There is also a great opportunity to commercialize these platforms for in vitro applications, such as drug screening and discovery for human diseases in actual development pipelines.
Currently, complex systems used for research applications and the elucidation of cellular mechanisms dominate the literature; however, widespread industrial adoption requires simple, cost-effective platforms that recapitulate the fundamental functions and response of the tissue [18]. A major inhibitor to the development of innovative and effective therapeutics is the enormous cost of drug development. A 2016 study concluded that the cost of drug development per approved compound was upwards of $2.5 billion [286]. Organ-on-chip systems have the potential to reduce these costs by supplementing early in vitro tests used to characterize the toxicity of both the drug and its metabolites [287, 288]. Additionally, complex systems may be used to precede in vivo evaluation in large or small animal studies [21]. Animal models are relatively inexpensive in comparison to human clinical trials. However, conducting extensive animal tests with a large number of drug candidates can be costly—especially when most compounds are shown to be non-effective and fail in this stage of testing [289]. The massive investment into drug development demonstrates the need for new tools with better predictive capabilities to reduce attrition rates, pre-clinical costs, and time-to-market. While the adoption of organ-on-chip devices into the drug development process may not yield direct cost savings, it has the potential to reduce failures in animal studies and clinical trials by identifying ineffective or unsafe drugs earlier. By reducing the number of compounds that are subject to expensive animal and human studies, organ-on-chip systems can reduce the average cost of drug development [17].
Some companies have already showed interest in tissue-on-chip models [287]. However, there is a huge potential for wide commercial applications of such engineered tissues. To this end, we need sophisticated bioprinting techniques capable of biofabrication in a scalable, high-speed, accurate, and high-throughput manner. These techniques would be able to make heterogeneous and biomimetic tissue constructs. In addition, integrated bioprinted tissues with microfluidics should be miniaturized to minimize the time and cost of fabrication, while having proper tissue function and durability. In particular, fluidic systems will provide efficient transfer of drug molecules and their metabolites on tissue-on-chip models based on the tissue functionality in vivo. Advanced biosensing and diagnostic technologies can also be used to significantly increase the accuracy of prediction using tissue models [290, 291]. Microfluidics may enhance the capability of biosensors in capturing and measuring metabolites by providing a dynamic and controlled environment. Multiple miniaturized tissues may be connected on a single organ-on-chip model to mimic the complexity of tissue function and response. Advanced microfluidic technologies have enabled us to make dual- or multiple-organ-on-chip platforms with physiologically relevant flow rates and shear stress values [292]. The combination of these two technologies will lead to the next generation of complex, commercial devices for physiological research, diagnosis, and drug selection. This new development will have a large impact on patient healthcare, allowing for both personalized selection of healthcare regimens for common and orphan diseases.
Conclusions
In conclusion, advances in both bioprinting and fluidic systems have led to rapid advancements in the modeling of organ systems. While simple systems have been capable of modeling physiological responses and characteristics of the larger organ tissues, complexities of organ-on-chip systems have grown through the use of multiple cell types and spheroid cultures. The next generation of organ-on-chip systems will utilize the complementary strengths from bioprinting (high spatial resolution, reproducible with high fidelity) and microfluidics (physiological environment of mechanical and chemical gradients), along with the advantage of utilizing human or even patient specific cells. In combination, these systems have the possibility to overcome the translational challenges associated with animal testing, with the capability of being reproduced and manufactured at a scale that will allow for broad use.
Acknowledgments
The authors acknowledge funding from the National Institutes of Health (5R01AR057837-06, 1R01EB021857-02, 1R01AR066193-03, 1U01CA214411-01A1, 1R01GM126571-01, 1R01AR073135-01A1).