Abstract
Currently, numerous studies are conducted using nanofibers as a scaffold for culture cardiac cells; however, there still needs to be more research evaluating the impact of the physicochemical properties of polymer nanofibers on the structure and function of cardiac cells. We have studied how poly( -caprolactone) and polyurethane nanofibrous mats with different physicochemical properties influence the viability, morphology, orientation, and maturation of cardiac cells. For this purpose, the cells taken from different species were used. They were rat ventricular cardiomyoblasts (H9c2), mouse atrial cardiomyocytes (CMs) (HL-1), and human ventricular CMs. Based on the results, it can be concluded that cardiac cells cultured on nanofibers exhibit greater maturity in terms of orientation, morphology, and gene expression levels compared to cells cultured on polystyrene plates. Additionally, the physicochemical properties of nanofibers affecting the functionality of cardiac cells from different species and different parts of the heart were evaluated. These studies can support research on understanding and explaining mechanisms leading to cellular maturity present in the heart and the selection of nanofibers that will effectively help the maturation of CMs.
-caprolactone) and polyurethane nanofibrous mats with different physicochemical properties influence the viability, morphology, orientation, and maturation of cardiac cells. For this purpose, the cells taken from different species were used. They were rat ventricular cardiomyoblasts (H9c2), mouse atrial cardiomyocytes (CMs) (HL-1), and human ventricular CMs. Based on the results, it can be concluded that cardiac cells cultured on nanofibers exhibit greater maturity in terms of orientation, morphology, and gene expression levels compared to cells cultured on polystyrene plates. Additionally, the physicochemical properties of nanofibers affecting the functionality of cardiac cells from different species and different parts of the heart were evaluated. These studies can support research on understanding and explaining mechanisms leading to cellular maturity present in the heart and the selection of nanofibers that will effectively help the maturation of CMs.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Cardiovascular disease (CVD) is the most common cause of death worldwide [1]. CVD includes, among others, ischemic heart disease, which causes chronic hypoxia of cardiomyocytes (CMs), whereby more cells undergo permanent damage and apoptosis [1, 2]. It is still not an effective treatment that could help to regenerate damaged cardiac cells and restore the proper functioning of the heart. Therefore, searching for new treatments to help repair the heart is necessary. It is obligatory to develop new in vitro models that accurately mimic the structure and function of human myocardial tissue, which will facilitate the understanding of the mechanisms occurring in the heart [3–6]. The technique that can potentially treat heart diseases and mimic cardiac tissue is tissue engineering (TE). It is an interdisciplinary field of science that develops tissue-mimicking structures using cells, biocompatible materials, and suitable biochemical and physical factors. One of the elements often used in TE is a scaffold, which should provide mechanical and structural properties similar to the extracellular matrix (ECM) properties [7–9]. In addition, ECM mediates signals between cells and contains growth factors. The ECM in cardiac tissue consists of proteins and polysaccharides, which can be divided into glycoproteins, proteoglycans, and glycosaminoglycans. Glycoproteins include fibronectin and laminin, as well as collagen (COL) and elastin, which give the ECM its elasticity. The ECM mediates signals that regulate the function of CMs, affecting the growth, differentiation, adhesion, and proliferation of cells [8].
Nanofibers are often used in TE for in vitro cardiac models as scaffold [10]. Nanofibers are defined as one-dimensional, elasticity, solid-state nanomaterials, with a diameter of up to 1000 nm (due to e.g. high porosity and textile production) [11]. Nanofibers show unique tensile and compressive strength. Therefore, they have appropriate elasticity to allow CM contraction. The nanofibers form three-dimensional (3D) structures with a large surface-to-volume ratio, the possibility of surface functionalization, high availability of various materials, porous (based on the literature, the porosity of nanofibers refers to the presence of empty spaces within the structure of nanofibrous mats [7]), gas-permeable, and an ECM-like environment. Thus, it is possible to utilize nanofibrous materials as scaffolds for cells in cardiac tissue in vitro models [10–13].
A suitable production method should be selected for nanofibrous materials to have appropriate structural properties and provide an adequate environment for cell culture. Electrospinning (ES) and solution blow spinning (SBS) are often used to fabricate nanofibrous mats for cardiac cell models. ES is simple, cheap, and can be applied to various polymers; however, the method has low production efficiency and requires high voltage (up to 60 kV) to generate the driving force to form fibers [14]. The SBS is a technique that was used for the first time in 2009. In SBS, nanofibers are fabricated by using a system of concentric chambers of a nozzle. An inner chamber blows out polymer solution, and from the outer chamber flows out a stream of pressurized gas (such as air, nitrogen, and argon). The gas stream is blown at high speed causing the polymer to stretch and the solvent to evaporate. Nanofibers are collected by the collector. Nanofibers produced by SBS have great potential for use due to the simplicity of production and application to various polymers. In addition, compared to ES, using SBS allows similar control over the nanofibers by obtaining specific physical properties (nanofiber size, pore size, fibers orientation) with a more efficient production rate in safer equipment. Nanofibrous materials produced by SBS can have higher porosity and elasticity than those fabricated by ES. Additionally, the SBS has excellent potential for industrial use [14–18].
The literature presents different nanofibrous materials used as a scaffold for cell culture, including cardiac cells. In the production of nanofibers, biodegradable polymers such as polyurethane (PU) and poly( -caprolactone) (PCL) in composite or with surface modification are most commonly used. Most investigations focus on studying the influence of nanofibrous structure on cardiac cell attachment, viability, maturation, morphology, and orientation [19–35]. Tomecka et al compared the viability and alignment of rat cardiomyoblasts (H9c2), neonatal rat CMs (NRCMs), and human CMs, which were cultured on PU nanofibrous materials modified by various proteins (such as COL, poly-l-lysine, gelatin, laminin, and fibronectin). According to results for all cell types, cells grown on the modified nanofibrous materials had a more elongated shape, were more anisotropic, and had higher viability than cells cultured on polystyrene plates (PS) [19]. Other researchers, Karimi et al, modified PCL nanofibers by using alginate and graphene oxide. The nanofiber surface became more hydrophilic, which provided higher cell viability and attachment of human cardiac progenitor cells cultured on nanofibrous materials than cultures grown on the PS [20]. Other research describes using composite nanofibers with PU, chitosan (Cs), and carbon nanotubes. An increase in the viability of rat cardiomyoblasts (H9c2) and human umbilical vein endothelial cells was observed for composite nanofibers compared to PU nanofibers without other components [21]. Also, composite nanofibers with conductive properties have been studied so far [22–24]. For example, conductive polypyrrole (PPy) encapsulated silk fibroin nanofibers were used to culture mouse CMs (HL-1), NRCMs and human-induced pluripotent stem cell-derived CMs. The result confirmed that the electrical properties of nanofibers influence the expression of proteins specific to mature heart cells (i.e. α-actinin, connexin 43) [25]. Depending on the methods and parameters of fabrication of nanofibrous mats, random or parallel alignment of fibers could be obtained [26–29]. Cesur et al compared how random and aligned polylactic acid (PLA)/polyethylene glycol/COL nanofibers affect the orientation and morphology of H9c2 cells [30]. Cells cultured on parallel nanofibers have been shown to exhibit more significant proliferation, elongated morphology, and parallel orientation than cells cultured on random nanofibers and the PS. It was also confirmed that primary CMs isolated from neonatal rats cultured on aligned PLA/Cs nanofibers have parallel orientation and a more elongated, rod-like shape than cells cultured on random nanofibers. In addition, an increase of expression of cardiac-specific proteins such as α-actinin and troponin I, was noticed [31]. Moreover, the potential use of nanofibrous materials for the differentiation and maturation of stem cells into mature CMs was reported [32, 33]. Conductive biopolymeric Cs/polyvinyl alcohol/multi-wall carbon nanotubes nanofibers were used to differentiate unrestricted somatic stem cells (USSCs) into cardiac cells. The differentiation of USSCs into CMs was confirmed by increased expression of cardiac-specific genes such as β-MHC, cTnI, and contractile-related protein—Cx43 [34]. In turn, Torabi et al confirmed that nanofibers made of poly(lactic-co-glycolic acid) with platelet-rich plasma stimulate the differentiation of iPSC into iPSC-CMs [35].
-caprolactone) (PCL) in composite or with surface modification are most commonly used. Most investigations focus on studying the influence of nanofibrous structure on cardiac cell attachment, viability, maturation, morphology, and orientation [19–35]. Tomecka et al compared the viability and alignment of rat cardiomyoblasts (H9c2), neonatal rat CMs (NRCMs), and human CMs, which were cultured on PU nanofibrous materials modified by various proteins (such as COL, poly-l-lysine, gelatin, laminin, and fibronectin). According to results for all cell types, cells grown on the modified nanofibrous materials had a more elongated shape, were more anisotropic, and had higher viability than cells cultured on polystyrene plates (PS) [19]. Other researchers, Karimi et al, modified PCL nanofibers by using alginate and graphene oxide. The nanofiber surface became more hydrophilic, which provided higher cell viability and attachment of human cardiac progenitor cells cultured on nanofibrous materials than cultures grown on the PS [20]. Other research describes using composite nanofibers with PU, chitosan (Cs), and carbon nanotubes. An increase in the viability of rat cardiomyoblasts (H9c2) and human umbilical vein endothelial cells was observed for composite nanofibers compared to PU nanofibers without other components [21]. Also, composite nanofibers with conductive properties have been studied so far [22–24]. For example, conductive polypyrrole (PPy) encapsulated silk fibroin nanofibers were used to culture mouse CMs (HL-1), NRCMs and human-induced pluripotent stem cell-derived CMs. The result confirmed that the electrical properties of nanofibers influence the expression of proteins specific to mature heart cells (i.e. α-actinin, connexin 43) [25]. Depending on the methods and parameters of fabrication of nanofibrous mats, random or parallel alignment of fibers could be obtained [26–29]. Cesur et al compared how random and aligned polylactic acid (PLA)/polyethylene glycol/COL nanofibers affect the orientation and morphology of H9c2 cells [30]. Cells cultured on parallel nanofibers have been shown to exhibit more significant proliferation, elongated morphology, and parallel orientation than cells cultured on random nanofibers and the PS. It was also confirmed that primary CMs isolated from neonatal rats cultured on aligned PLA/Cs nanofibers have parallel orientation and a more elongated, rod-like shape than cells cultured on random nanofibers. In addition, an increase of expression of cardiac-specific proteins such as α-actinin and troponin I, was noticed [31]. Moreover, the potential use of nanofibrous materials for the differentiation and maturation of stem cells into mature CMs was reported [32, 33]. Conductive biopolymeric Cs/polyvinyl alcohol/multi-wall carbon nanotubes nanofibers were used to differentiate unrestricted somatic stem cells (USSCs) into cardiac cells. The differentiation of USSCs into CMs was confirmed by increased expression of cardiac-specific genes such as β-MHC, cTnI, and contractile-related protein—Cx43 [34]. In turn, Torabi et al confirmed that nanofibers made of poly(lactic-co-glycolic acid) with platelet-rich plasma stimulate the differentiation of iPSC into iPSC-CMs [35].
Based on the above works, it can be concluded that various investigations are carried out using nanofibrous materials as scaffolds in vitro models to find the structure which mimics structurally and functionally the heart's ECM and help to explain the effect of the structure on the cardiac tissue. This work aimed to study the viability, orientation, morphology, and maturation of cardiac cells derived from different species and other parties of the heart cultured on specifically designed nanofibrous materials. In the context of biological research, the investigation of three different species can show the difference between species and the difference between cell lines from the ventricle and the atrium. Additionally, it was tested how the physicochemical properties of nanofibrous materials, such as polymer type, fiber diameter, and elasticity, affect the functioning of cardiac cells. So far, in vivo rat and mouse models are the most widely used research models to help understand how cardiac tissue works. However, these are not adequate models for research on finding an effective treatment method for human diseases because there are differences between species that are not fully explained and understood [36]. We studied how aligned PCL and PU nanofibers influence the cell culture of rat ventricular cardiomyoblasts (H9c2), immortalized mouse atrial CMs (HL-1), and primary human ventricular CMs (HCMs). According to the best of our knowledge, this is the first report comparing how various physicochemical properties of nanofibrous materials affect the viability, orientation, morphology, and maturation of cardiac cell lines isolated from different species. It could help understand and explain differences between cellular mechanisms in rodent and human cardiac cells and support research into the selection of nanofibers that effectively improve the function of cardiac cells in in vitro models.
2. Materials and methods
2.1. Fabrication of PCL and PU nanofibers
To produce nanofibers from PCL (Sigma Aldrich Mn = 80 000) and PU (ChronoFlex C75D, AdvanSource Biomaterials), the SBS technique was utilized. PCL was dissolved in 2-2-2-trifluoroethanol (>99%, ABCR) at a concentration of 8% and 10% (% w/w). PU solutions in 1,1,1,3,3,3-hexafluoroisopropanol (>99%, ABCR) were prepared at a concentration of 5% and 5.5% (% w/w). Polymer solutions were stirred overnight at room temperature to ensure the appropriate polymer chain relaxation leading to spinnable rheological properties of solutions. The procedure of the fabrication of nanofibers using SBS was described in detail previously [37, 38]. The following process parameter values were used: the working distance between the nozzle and collector: 50 cm, polymer solution flow rate: 30 ml h−1, compressed gas pressure: 1 bar, and rotational speed of the cylindrical collector: 15 000 rpm. The used collector rotational speed allowed to obtain aligned fibers.
2.2. Characterization of PCL and PU nanofibers
2.2.1. Material morphology
A scanning electron microscope (SEM, Phenom G1, PhenomWorld) was utilized to evaluate the nanofibers' diameter and orientation. Nanofibers were imaged in 2500×, 5000×, 7500×, and 10 000× magnifications. N = 100 nanofiber diameters for every type of material were measured using Fiji software [39] to obtain the average fiber diameter. To define fiber orientation, average fiber deviation from alignment direction was measured. As an alignment direction, the line perpendicular to the bottom edge of the SEM image was assumed. N = 100 angles between the fiber and the line were measured. Our previous research described the porosity of nanofiber's in detail [19].
2.2.2. Mechanical properties
The universal testing machine (Instron 3345, Instron) was used to examine the materials' tensile strength. For each polymer, three different nanofibrous mats were used. 5 samples with 5 mm width and 20 mm of testing length were acquired from each type of nanofibrous mat. Next, the samples were stretched at the stretching speed of 5 mm min−1. The mechanical strength of the nanofibrous materials was measured in two directions—in the parallel (PL) and perpendicular (PP) direction to fibers arrangement.
2.3. Cell culture
Three different cardiac cell lines were used in the experiments: rat cardiomyoblasts (H9c2 cell line, ATCC), derived from embryonic myocardium; primary human CMs (HCMs cell line, ScienCell), derived from ventricles of the adult heart; and mice immortalized CMs HL-1 (Sigma-Aldrich) derived from the atrial tumor. H9c2 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Sigma-Aldrich) supplemented with 10% v/v fetal bovine serum (FBS, Gibco), 1% v/v 100 mM penicillin–streptomycin (Sigma-Aldrich) and 1% v/v 200 mM L-glutamine (Sigma-Aldrich). HCM cells were maintained in DMEM: Nutrient Mixture F-12 (DMEM/F12, Gibco) supplemented with 10% v/v (FBS (Gibco), 1% v/v 100 mM penicillin–streptomycin (Sigma-Aldrich), 1% v/v 200 mM L-glutamine (Sigma-Aldrich), 1% v/v 100 mM sodium pyruvate (Sigma-Aldrich), 1% v/v cardiac myocyte growth supplement (CMGS, ScienCell) and 0.01% v/v MEM non-essential amino acids (NEAA, Sigma-Aldrich). HL-1 cells were cultured in Claycomb Medium (Sigma-Aldrich), supplemented with 10% v/v FBS (Gibco), 1% v/v 100 mM penicillin–streptomycin (Sigma-Aldrich), 1% v/v 200 mM L-glutamine (Sigma-Aldrich) and 1% v/v 10 mM norepinephrine. The cultures were maintained in a humidified incubator (37 °C, 5% CO2, HERA-cell 150, Thermo Scientific). Monolayer cell cultures were washed with phosphate buffered saline (PBS, Sigma-Aldrich) and trypsinized with 0.25% Trypsin (Sigma-Aldrich) to prepare cell suspensions. Then the cells were resuspended in a culture medium. Before cell seeding, PS were covered by 0.01% poly-l-lysine solution (ScienCell), fibronectin solution (Sigma-Aldrich), and gelatin (Sigma-Aldrich) (5 µg ml−1 fibronectin in 0.2 mg ml−1 gelatin solution) for HCM and HL-1 cells, respectively. They were incubated for 24 h (37 °C, 5% CO2).
2.4. Cell culture on nanofibrous mats
Nanofibrous mats were cut by laser (Universal VLS2.30DT) (laser power 17%, speed 13%, 800 PPI) in a circle shape with a 9 mm diameter, and they were placed in a 24-well plate. Unique polycarbonate (PC) holders were fabricated and placed on the nanofibrous mats in culture wells. The holders adjusted to the size of the culture well, which is used to prevent the nanofibrous mats from flowing out. Thanks to them, the nanofibrous mats were placed at the bottom of the 24-well plate during the culture. Next, the nanofibrous mats were sterilized with 70% EtOH (POCH) solution for 30 min and dried in an oven at 40 °C (Binder). The surface of nanofibrous materials was modified with oxygen plasma (0.3 mbar, 90 s, Diener) to improve their hydrophilic properties. Then, the nanofibrous mats were coated with protein solutions to increase cell adhesion and incubated for 24 h. Nanofibrous mats used to culture H9c2 and HCM cells were coated with 400 µl of 0.01% poly-l-lysine solution (ScienCell). In contrast, nanofibrous mats used for the culture of HL-1 cells were coated with 400 µl of 5 µg ml−1 fibronectin in 0.2 mg ml−1 gelatin solution (Sigma-Aldrich). Twenty-four hour after that, the protein solutions were removed. The nanofibrous mats were washed with a culture medium, and the cells were seeded on nanofibrous mats. Then, H9c2, HCM, and HL-1 cells were seeded on nanofibrous mats with a density of 3.3  104 cells cm−2, 6.6
104 cells cm−2, 6.6  104cells cm−2, and 6.6
104cells cm−2, and 6.6  104 cells cm−2, respectively. The culture medium was exchanged every other day for H9c2 and HCM cells and daily for HL-1 cells. Cell culture on polystyrene well was used as a control.
104 cells cm−2, respectively. The culture medium was exchanged every other day for H9c2 and HCM cells and daily for HL-1 cells. Cell culture on polystyrene well was used as a control.
2.5. AlamarBlue assay
AlamarBlue assay (Bio-Rad) allows quantitative evaluation of cell viability. After aspiration medium from cell cultures, 500 µl of 10% alamarBlue solution (diluted in FBS-free medium) was added and incubated at 37 °C for 3 h. The intensity of the fluorescence signal was measured in a plate reader (SpectraMax iD3, Syngen) at an excitation wavelength of 552 nm and an emission wavelength of 582 nm. The dye used in the alamarBlue assay is non-toxic to the cells. The alamarBlue assay allows comparing the fluorescence signal values obtained by cells cultured on a polystyrene plate (control) with fluorescence signal values achieved by cell cultures grown on nanofibrous mats. AlamarBlue assay was performed after 24 h and 10 d of H9c2, HL-1, and HCM cell seeding.
2.6. Calcein-AM (CAM) assay
To observe cell alignment, CAM assay was performed. CAM (1 mg ml−1 in anhydrous DMSO, Sigma-Aldrich) dye was diluted to 0.5 µg ml−1 in a medium and then carried out to perform microscopic observations (Nikon Eclipse Ts2-FL) of living cells after 24 h and 10 d of culture for investigated cell lines. CAM is a non-fluorescent dye that can passively permeate the cellular membrane of living cells. CAM is converted by esterases to calcein, which gives green fluorescence at 470–510 nm wavelength. Then, based on the images, an analysis of cell morphology and cell orientation was conducted using the ImageJ program.
2.7. Immunofluorescence staining of cardiac cells
The cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) in PBS (Thermo Fisher Scientific) for 10 min at room temperature (RT). Next, the cells were permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) in PBS. After that, 2.4% bovine serum albumin (Thermo Fisher Scientific) solution was utilized to reduce non-specific hydrophobic binding (blocking serum) (50 min, RT), and then the cells were washed with 0.1% Triton X-100. The cells were incubated with primary antibody at 4 °C overnight: rabbit monoclonal anti-troponin T (1:100) (Abcam), and mouse monoclonal anti-α-actinin (1:100) (Sigma-Aldrich), or rabbit polyclonal anti-MYH6 (1:100) (Proteintech) and mouse monoclonal anti-MYH7 (1:100) (Santa-Cruz Biotechnology). Then, the cells were washed with 0.1% Triton X-100, and they were incubated with secondary antibody: goat anti-rabbit Alexa Fluor 488 (1:200) (Thermo Fisher Scientific) and goat anti-mouse Alexa Fluor 594 (1:200) (Thermo Fisher Scientific) for 1 h. All antibodies were diluted in a blocking solution. Next, the cells Hoechst 33342 (10 μg ml−1 in PBS) (Thermo Fisher Scientific) was added. After 5 min, the cells were washed with 0.1% Triton X-100. The stained cells were observed under Zeiss Axio Observer 7 + LSM 900 confocal microscope.
2.8. Measurements of cardiac genes by a real-time reverse transcription-quantitative polymerase chain reaction
RNA was extracted using RNeasy Mini Kit (Qiagen) and then reverse transcribed into cDNA using RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). RT-PCR (quantitative real-time polymerase chain reaction) was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) on a CFX Connect Real-Time PCR System (Bio-Rad) using GAPDH as a housekeeping gene. Specific cardiac genes whose expression changes with CM maturation were selected for study (table S1 in supplementary information). The relative expression levels of each gene were carried out in triplicate. It was normalized to the housekeeping gene's expression and calculated using the ΔΔCT method.
2.9. Statistical analysis
The quantitative results were expressed as the mean ± standard deviation (SD) based on three independent experiments using the OriginPro 8 program. Statistical analysis was performed using one-way analysis of variance (ANOVA) or Student's t-tests depending on the experimental design. Values of p < 0.05 was considered statistically significant and marked with an asterisk.
3. Results
3.1. Structural properties of nanofibers
The SBS technique was used to make PCL and PU nanofibrous mats. Images of nanofibers' structure taken using a SEM are shown in figure 1. Four types of nanofibers were fabricated: PCL nanofibers (1) with an average diameter of 250 ± 78 nm, PCL nanofibers (2) with an average diameter of 509 ± 178 nm, PU nanofibers (1) with an average diameter of 279 ± 108 nm, PU nanofibers (2) with an average diameter of 452 ± 151 nm (all parameters for nanofibrous mats are shown in figure 1). As expected, the average size of the nanofibers within the materials depended on the initial concentration of the polymer in the solution. It can be noticed that nanofibers have a parallel arrangement and form tightly packed bundles. In figure 1(B), PU nanofibers are more arrangement (for PU (1), it is 61%, for PU (2), it is 50%) than PCL nanofibers (for PCL (1) it is 42%, for PCL (2) it is 45%) for the measurements from −15° to +15°. However, the changes are not statistically significant differences. In figure 1(C), one can see the high porosity of the materials used (between 80% and 90%). The nanofibers with smaller diameters have a higher porosity (PCL (1) is 90%, PU (1) is 86%, while PCL (2) has a porosity of 83% and PU (2) is 82%). PCL (2) and PU (2) nanofibers have similar porosity, while the other nanofiber types have statistically significant differences.
Figure 1. (A) SEM images of aligned PCL nanofibers with an average diameter of 250 ± 78 nm, PCL nanofibers with an average diameter of 509 ± 178 nm, PU nanofibers with an average diameter of 279 ± 108 nm, PU nanofibers with an average diameter of 452 ± 151 nm, (B) number of nanofibers in relation to the angle of the alignment, (C) porosity measurements of PCL and PU nanofibers mats. *- p < 0.05- statistically significant differences were determined based on one-way ANOVA. n ⩾ 3.
Download figure:
Standard image High-resolution image3.2. Mechanical characterization of PCL and PU nanofibers
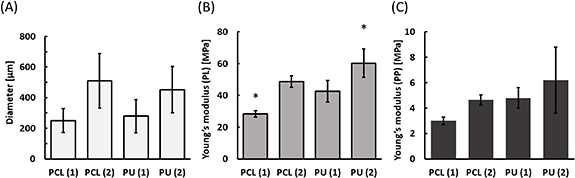
The mechanical properties of the nanofibers are presented in figure 2, such as diameter and Young modulus in the parallel (PL) and perpendicular (PP) direction to fibers arrangement. Young's modulus described the elasticity of the materials. The Young modulus for PCL (2) nanofibers in the PL direction was 48.6 MPa, and in the PP direction was 4.6 MPa. A similar Young modulus for PU (1) nanofibers was noticed. It equals in PL direction was 42.6 MPa and PP direction was 4.8 MPa. Nanofibers with the highest stiffness for both PL and PP are PU nanofibers of larger diameter, while PCL nanofibers of smaller diameter are the most elastic. The results obtained for all types of nanofibers for the tensile strength measurements showed statistical differences only for PL measurements. For PL measurements, statistically significant differences were observed between all types of nanofibers except between PCL (2) and PU (1) nanofibers. The difference in Young modulus between PP and PL tests indicates that the preferred orientation of the nanofibers within the mats was achieved. The fabricated nanofibrous materials with different physicochemical properties (polymer, diameter, Young modulus) were used to evaluate the impact of the parameters on the functioning of cardiac cell cultures.
Figure 2. Physical properties of polycaprolactone (PCL) and polyurethane (PU) nanofibers. (A) Diameter of nanofibers, (B) and (C) the tensile strength was measured parallel (PL) and perpendicular (PP) to the fiber orientation. For all types of nanofibers for the tensile strength measurements, the case of PL measurements shows statistically significant differences (p < 0.05) than in the case of PP, which they do not. For PL measurements, significant statistical differences were achieved between all types of nanofibers except for between PCL (2) and PU (1). *-p < 0.05- statistically significant differences were determined based on one-way ANOVA. n ⩾ 3.
Download figure:
Standard image High-resolution image3.3. Cardiac cell growth on nanofibrous mats
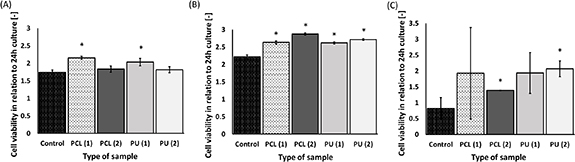
The viability of rat cardiomyoblasts (H9c2), mice CMs (HL-1), and HCMs are presented in figure 3. For rat's CMs, cell viability increased for both polystyrene plates and nanofibrous mats after 10 d-culture. A statistically significant increase was achieved by the cultures grown on PCL (1) nanofibers with an average diameter of 250 ± 78 nm, and PU (1) nanofibers with an average diameter 279 ± 108 nm compared to the 10 d-culture on polystyrene plate (control). The highest viability of the cells was noticed for PCL (1) (2.15 times higher than cell culture after 24 h) on the 10th day of culture. The lowest viability was observed for the cells cultured on PS (control) (1.74 times higher than cell culture after 24 h).
Figure 3. Cell viability for H9c2 (A), HL-1 (B) and HCM (C) cultured on nanofibers after 10 d in relation to 24 h culture. *—p < 0.05—statistically significant increase in cell viability was determined by Student's t-test compared cultures grown on nanofibers to cultures grown on polystyrene plates (control) after 10 d-culture. n = 3.
Download figure:
Standard image High-resolution imageBased on the alamarBlue assay for mice's CMs (HL-1 cells), it was concluded that cell viability increased for nanofibers and polystyrene plates (control). According to figure 3(B), the increase in cell viability for cultures grown on nanofibers after 10 d was higher than for the control; mainly, it was noticed for cells cultured on PCL (2) nanofibers.
According to figure 3(C), the viability of HCMs increased with time for every type of nanofibrous material used in this research. After 10 d of culture, a statistically significant increase in the viability of cells cultured on PCL (2) nanofibers with an average diameter of 509 ± 178 nm and PU (2) nanofibers with an average diameter of 452 ± 151 nm was determined compared to the control. Cell viability for control decreased after 10 d (0.19 times less than cell cultures after 24 h). The highest viability was noticed for PCL (2) nanofibers (1.39 times higher than cell viability for 24 h) on the 10th day of culture.
Based on the results presented in figure 3, HL-1 cells are the only one of the three cell lines to give a statistical increase in viability for cultures grown on all types of nanofibers compared to control after 10 d. However, for H9c2, HL-1, and HCM cells, the increase after 10 d cultured was noticed.
3.4. H9c2, HL-1, and HCM cell alignment on nanofibrous mats
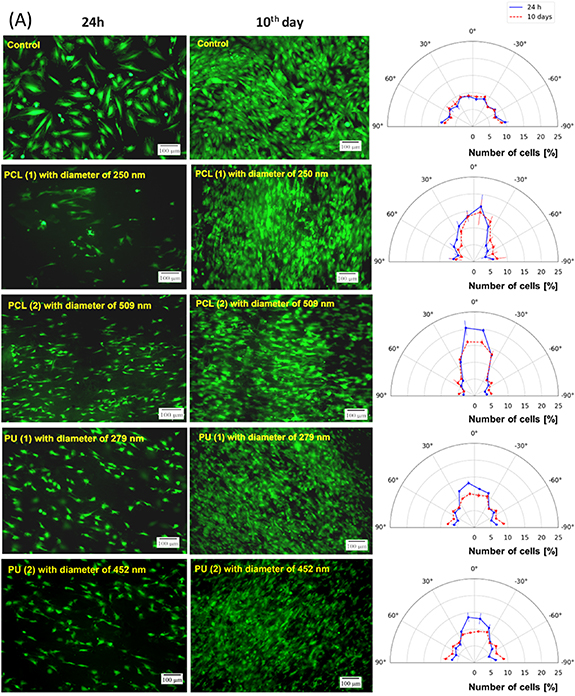
Cell staining with CAM was performed to study the cell alignment of H9c2, HL-1, and HCM cells. Based on figure 4 and table 1, it can be concluded that H9c2, HL-1, and HCM cells have parallel orientations on aligned nanofibers, while cells grown on the PS (control) were oriented randomly. The distribution of H9c2, HL-1, and HCM cells grown on polystyrene was uniform for each direction. The total number of cells was between 14% and 18% (from −15° to +15°) and 31%–37% (from −30° to +30°). Based on microscopic observation and image analysis, it was noticed that for the measurements from −15° to +15°, the most significant number of H9c2 and HCM cells showed parallel arrangements for PCL (2) nanofibers, whereas HL-1 cells for PU (2). For 24 h culture, it equaled 40% (H9c2), 26% (HL-1), and 32% (HCM), and for 10 d culture, it was 32% (H9c2), 22% (HL-1), and 43% (HCM). It gave about twice the number of cells with parallel arrangement compared to the control (2.4—H9c2, 1.9—HL-1, and 2.4—HCM cells). The same tendency was noticed for the arrangement of the cells between the angle of −30° and +30°. Furthermore, it was observed that during 10 d of culture, the number of parallel cells decreased for H9c2 (1.8 compared to the control) and HL-1 (1.4 compared to the control) cells, whereas it increased for HCM (2.7 compared to the control) cells.
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageFigure 4. The (A) H9c2, (B) HL-1, and (C) HCM cells stained with calcein-AM (CAM) (green color), cultured for 24 h and 10 d on different nanofibrous mats. A polystyrene plate was used as a control. On the right side are graphs showing the alignment of the cells. The orientation of nanofibers was assumed to the 0°. Scale bars 100 µm.
Download figure:
Standard image High-resolution imageTable 1. Number of H9c2, HL-1, and HCM cells with parallel orientation cultured on nanofibers and polystyrene plate (control). *—p < 0.05—statistically significant differences were determined using the Student's t-test. n ⩾ 3.
| % Cells | |||||
|---|---|---|---|---|---|
| Surface | From (-) 15° to 15° | From (-) 30° to (-) 15° and from 15° to 30° | |||
| 24 h | 10th day | 24 h | 10th day | ||
| Control | H9c2 | 17 | 18 | 18 | 19 |
| HL-1 | 14 | 16 | 17 | 17 | |
| HCM | 17 | 16 | 16 | 15 | |
| PCL (1) | H9c2 | 30* | 28 | 23* | 23 |
| HL-1 | 22 | 20* | 21* | 21* | |
| HCM | 29 | 37 | 20 | 20 | |
| PCL (2) | H9c2 | 40* | 32* | 24* | 25* |
| HL-1 | 24* | 20* | 23 | 20* | |
| HCM | 32* | 43* | 19 | 23 | |
| PU (1) | H9c2 | 25* | 20 | 23* | 19 |
| HL-1 | 24* | 22 | 22* | 21* | |
| HCM | 31 | 25 | 21 | 22 | |
| PU (2) | H9c2 | 27* | 18 | 21 | 19 |
| HL-1 | 26* | 22* | 24* | 22* | |
| HCM | 16 | 22 | 16 | 21 | |
Additionally, cell morphology analysis was performed. The literature shows mature CMs have large, anisotropic, rod-like, elongated shapes (high length/width ratio and less roundness). Immature CMs are round, smaller than adult CMs, and were oriented randomly [40]. It was studied that H9c2, HL-1, and HCM cells grown on PS have a larger area than those cultured on nanofibrous mats (table 2). Moreover, cells grown on nanofibers have a higher length/width ratio and less roundness than cells grown on polystyrene plates. It can be noticed that the H9c2 cells grown on PCL with a larger diameter have the most rod-like shape (5.8 length/width ratio and 20.0% roundness, whereas for the control length/width ratio was 2.1 and roundness was 61.2%). In turn, the most rod-like shape of HL-1 cells was noticed for PU (2) nanofibers (2.4 length/width ratio and 51.7% roundness, whereas for the control, they equaled 1.8 and 56.6%). HL-1 cells are less elongated than H9c2 and HCM cells (table 2(B)). HCM grown on PCL nanofibrous mats have a higher length/width ratio and the lowest roundness (for PCL (1) 5.9 length/width ratio and 18.9% roundness; for PCL (2) 5.7 length/width ratio and 19.7% roundness) (table 2(C)) than HCM cultured on PU nanofibers and the PS (4.0 length/width ratio and 29.2% roundness). Based on these results, it can be concluded that cells grown on nanofibers with a larger diameter indicate a more significant anisotropy, rod-like, elongated shape than cells grown on nanofibers of smaller diameter and control. This made them more like mature CMs present in myocardial fibers.
Table 2. Morphological parameters of H9c2 (A), HL-1 (B), and HCM (C) cells. *—p < 0.05—statistically significant changes compared with control (the Student's t-test). The analysis was performed for 10-day culture. n ⩾ 3.
| Surface | Length (μm) | Width (μm) | Length/Width ratio | Area (μm2) | Roundness (%) |
|---|---|---|---|---|---|
| (A) | |||||
| Control | 42.0 ± 6.9 | 21.1 ± 5.5 | 2.1 ± 0.8 | 895.3 ± 217.8 | 61.2 ± 17.3 |
| PCL (1) | 42.8 ± 9.5 | 12.1 ± 1.8 | 3.7 ± 1.1* | 373.9 ± 92.1 | 29.4 ± 9.3* |
| PCL (2) | 74.8 ± 14.4 | 14.3 ± 4.5 | 5.8 ± 2.2* | 755.5 ± 236.2 | 20.0 ± 5.8* |
| PU (1) | 36.6 ± 6.5 | 14.4 ± 3.5 | 2.7 ± 0.7 | 637.6 ± 254.9 | 50.3 ± 18.1* |
| PU (2) | 29.8 ± 7.7 | 12.1 ± 3.3 | 2.7 ± 1.2 | 434.0 ± 139.0 | 50.0 ± 19.7* |
| (B) | |||||
| Control | 28.8 ± 9.2 | 16.3 ± 4.0 | 1.8 ± 0.7 | 355.1 ± 162.6 | 56.6 ± 14.5 |
| PCL (1) | 28.8 ± 6.1 | 16.7 ± 3.7 | 1.8 ± 0.4 | 364.5 ± 139.3 | 59.1 ± 12.8 |
| PCL (2) | 32.6 ± 10.8 | 15.9 ± 3.3 | 2.1 ± 0.7 | 382.7 ± 162.4 | 51.9 ± 17.3 |
| PU (1) | 31.9 ± 8.9 | 16.2 ± 4.6 | 2.1 ± 0.7 | 401.0 ± 131.2 | 51.7 ± 15.7 |
| PU (2) | 31.1 ± 8.0 | 13.9 ± 3.0 | 2.4 ± 1.0* | 313.9 ± 84.5 | 47.5 ± 17.0 |
| (C) | |||||
| Control | 109.3 ± 41.4 | 28.0 ± 6.5 | 4.0 ± 1.5 | 2189.5 ± 967.6 | 29.2 ± 14.3 |
| PCL (1) | 103.3 ± 32.5 | 18.2 ± 3.9 | 5.9 ± 2.2* | 1217.5 ± 500.4 | 18.9 ± 8.0* |
| PCL (2) | 125.0 ± 44.3 | 23.7 ± 7.9 | 5.7 ± 2.5* | 1844.2 ± 727.5 | 19.7 ± 10.1* |
| PU (1) | 91.2 ± 22.4 | 23.1 ± 15.5 | 4.7 ± 1.9 | 1417.8 ± 727.5 | 23.1 ± 8.7 |
| PU (2) | 91.5 ± 32.9 | 23.6 ± 7.5 | 4.0 ± 1.1 | 1490.1 ± 649.0 | 25.6 ± 8.3 |
3.5. Analysis of the maturation of cardiac cells
Genes and proteins specific to mature CMs were determined using immunofluorescence staining and RT-PCR techniques. The presence of CM-specific proteins, such as α-actinin (labeled in red) and troponin T (marked in green), MYH6 (marked in green) and MYH7 (marked in green) are shown in figures 5 and 6. H9c2 cells cultured on nanofibers showed increased α-actin expression compared to cells cultured without nanofibers. HL-1 cells cultured on nanofibers showed increased expression of α-actinin and troponin T compared to those cultured on the PS plate (control). The highest expression of both proteins was obtained for cells cultured on PCL (1), PCL (2), and PU (2) nanofibers with a diameter of 250 nm, 509 nm, and 452 nm, respectively.
Figure 5. Immunofluorescence staining of α-actinin (Alexa Fluor 594, red fluorescence), troponin T (Alexa Fluor 488, green fluorescence), and nucleus (Hoechst 33342, blue fluorescence) in H9c2 (A), HL-1 (B), and HCM (C) cells. Immunofluorescence staining was performed after 10 d of culture on a polystyrene plate (control) and PCL and PU nanofibrous mats. Scale bar 20 µm.
Download figure:
Standard image High-resolution imageFigure 6. Immunofluorescence staining of MYH7 (Alexa Fluor 594, red fluorescence), MYH 6 (Alexa Fluor 488, green fluorescence), and nucleus (Hoechst 33342, blue fluorescence) in H9c2 (A), HL-1 (B), and HCM (C) cells. Immunofluorescence staining was performed after 10 d of culture on a polystyrene plate (control) and PCL and PU nanofibers. Scale bar 20 µm.
Download figure:
Standard image High-resolution imageIn vivo, the myosin heavy chain (MHC) has isoforms whose expression depends on the maturation of cardiac cells [41]. In humans, there is the fetal α isoform (α-MHC, encoded by the MYH6 gene) and the mature β isoform (β-MHC, encoded by the MYH7 gene). During the maturation of HCMs, MYH7 expression increases significantly, while MYH6 shows a slight increase in expression. In rodent cells, it is the opposite; an increase in the expression of the isoform encoded by the MYH6 gene in relation to the isoform encoded by the MYH7 gene indicates the maturity of the cells [41].
The ratio of MYH6 and MYH7 proteins varies depending on the part of the heart [42]. Based on the literature, MYH7 is the main isoform of MHC in adult human ventricle. In contrast, the atrium expresses both isoforms; MYH6 and MYH7, but MYH6 expression is lower than MYH7. In the rodent heart is opposite; MYH6 is the major MHC isoform in the ventricle. In contrast, both isoforms are observed in the atrium, MYH6 and MYH7, but MYH7 expression is lower than MYH6 [41, 42]. Based on outcomes shown in figure 6, for H9c2 cells, MYH6 protein expression was observed. MYH7 protein is a trace amount in cells grown on PCL (1), PCL (2), and PU (1) nanofibers. For HCM cells, the difference in the level of expression MYH6 for cultures from control and nanofibers is not noticeable; however, the expression of MYH7 in cells grown on nanofibers is higher than in cells cultured on a polystyrene plate. Whereas for atrial HL-1 cells grown on nanofibers show increased expression of MYH7 compared to cells grown in control. Based on the obtained results, we can conclude that isoform MYH6 and MYH7 switching for H9C2, HL-1 and HCM cells are like those occurring in human and rodent cardiac cell maturation.
Based on all the above results, two types of nanofibrous mats, PCL (2) and PU (2), with diameters of ∼500 nm, were chosen for further research. RT-PCR technique was utilized to determine the level of gene expression, such as TNNT2 (troponin T), TNNI3 (troponin I), and SERCA2 (calcium-ATPase type 2) for H9c2 cells. For HL-1 and HCM CMs, genes such as ACTN2 (cardiac α-actinin), MYL2 (myosin regulatory light chain 2), and SCN5A (sodium voltage-gated channel alpha subunit 5) were additionally studied.
According to figure 7(A), the mean of expression of TNNT2 and SERCA2 genes is increased significantly for rat's cardiomyoblasts (H9c2) grown on PCL (2) nanofibers compared with cells cultured on polystyrene plate (control) and PU (2) nanofibers. The level of expression TNNT2 gene is 14.2 times higher for cells grown on PCL (2) than control. In the case of the TNNI3 gene, the highest level of expression was for cells grown on PU (2) nanofibers (3.2 times higher than the control). However, it is not statistically significant.
Figure 7. Quantitative gene expression analysis by qRT-PCR cardiac cells cultured on a polystyrene plate, PCL (2), and PU (2) nanofibrous mats for 10 d of cell culture. (A) troponin T (TNNT2), troponin I (TNNI3), calcium-ATPase type 2 (SERCA2) for H9c2, (B) troponin T (TNNT2), troponin I (TNNI3), cardiac α-actinin (ACTN2), calcium-ATPase type 2 (SERCA2), sodium channel protein type 5 subunit alpha (SCN5A), and myosin regulatory light chain 2 (MYL2) genes for HL-1 and HCM cardiomyocytes. *—p < 0.05—statistically significant differences were determined by comparison with the cells cultured on a polystyrene plate (control) (the Student's t-test). N = 3.
Download figure:
Standard image High-resolution imageFor HL-1 and HCM, most of the cardiac markers increased for cells grown on nanofibers compared to control (figure 7(B)). However, changes in gene expression were lower compared with H9c2 cardiomyoblasts. For HCM gene isoform of troponin T (TNNT2), which is mostly in fetal CMs, is decreased for cells grown on both types of nanofibers. In contrast, predominant in adult CMs isoform gene of troponin I (TNNI3) increased (figure 7(B)). For HCMs, two types of genes relating to the electrophysiology of the cells were chosen. For SERCA2 (1.2-fold of the control) and SCN5A (1.7-fold of the control) the highest level of expression was for HCM cells cultured on PCL (2) nanofibers. Whereas MYL2, which expression increases in ventricular human cell maturation, was 2.4 times higher for HCM cells grown on PU (2) nanofibers. For HL-1 cells, TNNT2, TNNI3, SERCA2, and SCN5A gene expression changes slightly for every type of nanofibrous materials. However, the mean of ACTN2 and MYL2 expression is higher for the cells grown on nanofibers than for the cells cultured on a polystyrene plate. HL-1 cells cultured on PCL (2) have the highest level of ACTN2 (3.7-fold of the control) and MYL2 (1.8-fold of the control).
4. Discussion
CVDs have the highest mortality rate among humans. The result is that research to find an effective treatment for heart disease is needed. Therefore, there is a need to continuously improve in vitro cellular models that will adequately mimic the complex working of cardiac tissue. A conventional 2D culture model using a polystyrene plate does not correspond to cardiac tissue's complex, oriented, and 3D structure. In the current research, most in vitro models use immature cardiac cells with different morphology and physiology than adult CMs. Therefore, they do not appropriately mimic the cardiac tissue's in vivo conditions. To imitate in vivo conditions, more and more studies are being conducted on a 3D model using nanofibers, which structural and mechanical are, like heart ECM. Additionally, based on previous studies, nanofibers affect cell arrangement, morphology, proliferation, and maturation [23–35, 43].
Nanofibers are widely used in cardiac in vitro models. However, it is still unknown how nanofibers' physicochemical properties should be selected, such as the size of diameter, porosity, elasticity, conductivity, and type of biomaterial, to mimic the structure of heart tissue. Nowadays, it is studied how the structure, materials composite, and modification of nanofibers influence cardiac cells adhesion, viability, orientation, and morphology [19–23, 44]. However, few studies compare nanofibers' effect with different physicochemical properties on cardiac cell functioning [19, 20, 34, 35]. We used alignment nanofibers made of biodegradable synthetic polyester such as PCL and biocompatible with heart tissue synthetic polymer such as PU fabricated using the SBS method. The SBS method was chosen because it is more efficient, cheaper, and safer than the commonly used ES technique. In addition, SBS allows to obtain nanofibers with structural and mechanical properties similar to the native ECM than the properties of nanofibers obtained after production by the ES method [14–19]. We have determined how different nanofibers 'properties influenced heart cells' orientation, morphology, viability, and maturation. In addition, it was investigated whether these properties had a different effect on different heart cell types taken from the heart ventricle or atrium. Scaffolds used in the TE of cardiac cells should have adequate elasticity because it impacts the morphology and contraction of myocardial cells.
The value of Young's modulus of the myocardium is in the range of 70–160 kPa (elasticity depends on the part of the heart) [45, 46]. The exact values vary with the location of the heart tissue, as well as the patient's age and state of health. Literature sources indicate that materials with Young's modulus ranging from 20 kPa to 92 MPa were used [45–47], and nanofibers whose elasticity was in this range were biocompatible with cardiac cells. However, which elasticity is the most appropriate for the TE of cardiac cells is still unknown. In this research, nanofibrous mats have four different elasticities (PCL (1) > PCL (2) ∼ PU (1) > PU (2)) (figure 2). However, significant differences were not observed in the phenotype of cardiac cells depending on the elasticity of these nanofibers. Such differences were observed in the case of the size of nanofibers. The structure of the ECM in the human cardiac tissue is built by fibers whose diameter is up to 300 nm [48, 49], and the most common range of diameter of nanofibers used in TE for cardiac cells is 200−700 nm [50, 51]. For comparison, the PCL and PU nanofibers were produced in two diameters similar to the diameter of fibers building the ECM heart in vivo. The PCL (1) and PU (1) nanofibers had a c.a. 250 nm diameter. On the other hand, PCL (2) and PU (2) nanofibers were around 500 nm. For all types of nanofibers, the increase in proliferation varied depending on the cell line. For H9c2 cells, the greatest increase in proliferation was observed on PCL (1) nanofibrous mats, for HL-1 on PCL (2), and for HCM on PU (2) nanofibers. Based on this, it is difficult to conclude which of the tested nanofibrous materials is the most universal for culturing different types of cell lines. It is worth mentioning that H9c2 are embryonic cells, HL-1 are immortalized cells that can proliferate indefinitely. At the same time, HCM are primary cells whose proliferation stops after a limited number of cell divisions, which may affect the results we obtained. Based on the results, it was concluded that PCL and PU nanofiber mats have no negative effect on the culture of cardiac cells, can stimulate their proliferation, and can be used to culture various types of cardiac cells [19, 27].
The cells imitate the structure of nanofibers and arrange in parallel to each other. As a result, the cells assume an elongated shape, which is more similar to the shape of CMs in vivo rather than the round shape of cells grown on the surface of a polystyrene plate [19, 26–29, 52]. Based on the results, PU nanofibers show a more parallel-oriented structure however, more mature morphology and parallel arrangement are shown cardiac cells grown on PCL nanofibers. The work also determined the effect of porosity nanofibers (PCL (1) > PU (1) > PCL (2) ∼ PU (2)), on cardiac cell behavior. Nanofibrous mats with lower porosity for a larger diameter have a greater effect on the maturation of cardiac cells than nanofibers with higher porosity. According to the results of viability, orientation, protein, and gene expression, the physicochemical parameters of nanofibers that have the greatest impact on the maturation and function of cardiac cells are the nanofiber's diameter, elasticity, and biomaterials. However, to this day, it has not been established how the biomaterial, elasticity, and size of diameters affect the cell's orientation, morphology, and maturation. In this study, it can be concluded that nanofibers 'three-dimensional structure increases the cultured cells' arrangement. In turn, the parallel orientation of the cells was not observed on the PS. The ventricular H9c2 and HCM cells, cultured on PCL nanofibers with a diameter of 500 nm, had the highest number of cells with elongated rod-like shapes (the high length/width ratio and low roundness) and with a parallel arrangement. Atrial HL-1 cells have the highest number of aligned cells with elongated, rod-shaped cells grown on nanofibers with a diameter of around 500 nm but made of PU. Based on these results, it can be concluded that all cell lines obtained anisotropic, rod-like shapes on nanofibers with a diameter of 500 nm. It was concluded that depending on the origin of the cells, selecting the appropriate biomaterial to produce nanofibers is also important.
According to the literature, to determine the maturity of CMs, it is not enough to assess the change in morphology and arrangement, but it is also necessary to examine the expression of specific cardiac genes and proteins whose expression increases or decreases with the maturation of heart cells [53]. So far, cardiac maturation was not studied using different nanofibers and cells (stem or cardiac) [25, 31–35]. We compared the impact of biomaterial, diameter, and elasticity on the maturation of cardiac cells from different species and part of the heart.
In vivo, TNNT2 is an isoform of troponin T, which increases during fetal development, decreases during the neonatal stage, and into adult CMs because isoform encoded by TNNT2 gene switch in isoforms encoded by TNNT3 or TNNT4 [54]. TNNT2 increased in H9c2 cells, which were cultured on PCL (2), and decreased in HCM cells which were cultured on PCL (2) and PU (2). H9c2 cells are not fully differentiated cells, and have been isolated from embryos, so they are more similar to fetal CMs than neonatal and adult CMs [41]. HCM cells are isolated from the human adult heart. A decrease in TNNT2 expression may mean that the cells mature [54]. Conversely, TNNI3 is an isoform of troponin I, which increases during the maturation of CMs and is the highest in adult cardiac cells in vivo [54, 55]. It was determined that ventricular HCM and H9c2 cells cultured on PCL (2) and PU (2) nanofibers had higher expression of TNNI3 than those cultured on polystyrene plates. A correlation between the TNNT2 and TNNI3 expression was observed, which is consistent with the literature data that the expression of the TNNT2 gene increases during cell differentiation and decreases in the further stages of CM development [41], at the same time, expression of TNNI3 should rise at a later stage of CM maturation [41, 54, 55]. Literature sources indicate an increase in troponin T and I for cardiac cells grown on various nanofibers [31, 35, 56, 57]. For example, Chen et al differentiated murine iPSC cells into CMs on PCL nanofibers. The results showed increased TNNT2 expression for cells cultured on nanofibers compared to cultures on a polystyrene plate [56]. Other research has shown an increase in the expression of troponin I in rat primary CMs grown on PLA/Cs nanofibers compared with cells cultured on PS. In conclusion, based on recent papers, H9c2, HL-1, and HCM are cultured on nanofibers, but research focuses on cell viability, adhesion, and morphology [21, 58, 59].
In human and rodent hearts, proteins, involved in the structure and function of cardiac tissue, can exist in different isoforms, and their expression levels differ between fetal and adult CMs. For example, the heavy chain of myosin with two isoforms, MYH6 and MYH7, builds thick filaments (myosin) of sarcomeres [41, 60]. MYH7 expression was upregulated in mature human ventricular CMs, whereas MYH6 gene expression was upregulated in mature rodent cardiac cells [41, 42]. Based on the results shown in figure 6, expression of MYH6 in H9c2 cells was observed, whereas expression of MYH7 was low. For HL-1 cells cultured on nanofibrous mats, the expression of MYH6 and MYH7 increased. These cells are from the atrium, and according to the literature, during the maturation of rodent atrial CMs, the expression of MYH6 and MYH7 is similar [41]. MYL2 is an isoform of the regulatory light chain of myosin. In vivo it increases significantly during maturation from the neonatal to the adult stage of ventricular human cardiac cells and from the postnatal to the adult stage for rodent cardiac cells [40, 61]. For HCM and HL-1 cells, an increase in MYL2 expression was noticed in cells cultured on both types of nanofibers (compared with control). For HCM cells, the highest expression of MYL2 was for PCL (2) nanofibers, while for HL-1 cells, for PU (2) nanofibers. Cardiac α-actinin is a protein that involves anchoring thin filaments (actin) and titin to Z-discs [40, 41, 62]. It was proved that ACTN2 participates in CM maturation, especially in the postnatal stage, although the mechanism is still unknown [62]. The increase in ACTN2 was noticeable for HCM and HL-1 cells, however, expression of cardiac α-actinin was higher in atrial CMs of mice cultured on nanofibers than in HCM cells. Also, previous studies confirmed that an increase in cardiac α-actinin may indicate the maturation of heart cells grown on nanofibers [25, 31].
During the development of the heart from the fetal to the adult stage, the electrophysiology of CMs is changed, which is based on the transport of sodium, potassium, and calcium ions [53–55, 60]. Calcium ions play an essential role in the contraction of CMs. This is related to a transient concentration of Ca2+ inside the CMs, which depends on the L-type calcium channels located in the sarcolemma and Ca2+ ions stored inside the cell. During the depolarization of the cell membrane, L-type channels are opened. Then, the influx of Ca2+ into the cells causes calcium ions to be released in the sarcoplasmic reticulum (SR) by ryanodine receptors sensitive to Ca2+. Calcium ions then bind to troponin C, which ultimately leads to contraction. During diastole, Ca2+ is transported from the cytosol to the SR by the ATPase SERCA2 (encoded by SERCA2) and into the extracellular space via the Na+/Ca2+ exchanger [54, 63]. In fetal CMs, the calcium-handling proteins (including SERCA2) are not developed; therefore, the contraction is slower and weaker than in the adult heart [54]. During the maturation of in vivo CMs from the fetal to the adult stage, there is a steady increase in the expression of SERCA2 [54, 63]. According to figure 7, in H9c2 cells grown on PCL (2), the expression of SERCA2 is the highest. In the case of primary HCM cells and immortalized HL-1 cells cultured on nanofibers, the SERCA2 level is the level is unchanged and like the control. According to these results, the increase of SERCA2 depended on the stage of cardiac cells development—the highest expression of SERCA2 during the maturation is for not fully differentiated H9c2 cells, less for immature HCM, and the least for HL-1 cells with mature electrophysiology. Another type of protein that regulates ion transport in cardiac cells is Nav1.5 (encoded by SCN5A). It is a voltage-gated sodium channel subunit. The expression also increases during the development of the heart [54, 63]. Based on the literature, it has been confirmed that nanofibers stimulate an increase in SERCA2 and SCN5A expression; however, the research was carried out on stem cells during the differentiation [64, 65].
Based on the immunostaining and RT-PCR results, it can be concluded that H9c2, HCM, and HL-1 cells grown on nanofibers are more mature than cells grown on a polystyrene plate. However, depending on the type of nanofibers (different in elasticity and biomaterial), they initiated a further increase or decrease in the expression of genes and their protein products specific for cardiac maturation. Thus, the physicochemical properties of the nanofibers influenced the maturation of human and rodent CMs or cardiomyoblasts originating from the ventricle or atrium.
PCL and PU nanofibers are widely used as scaffolds in TE for cardiac in vitro models. Both materials are biodegradable, low cost, non-toxic, and have appropriate mechanical strength and elasticity to culture cardiac cells [66, 67]. PCL nanofibers are characterized by better adaptation in vivo conditions, while PU nanofibers have higher tensile strength, toughness, and resistance against degradation [68]. However, both polymers are hydrophobic therefore, mostly they are utilized in composite, or it is necessary to modify the surface by oxygen plasma and protein solution such as gelatin, fibronectin, or poly-l-lysin. These solutions improve cell adhesions [66, 67]. In this study, PCL and PU nanofibers were utilized to determine which type of biomaterial can impact on functioning and stimulation of different maturation mechanisms for cardiac cells. It was noticed that cells isolated from the ventricle, such as H9c2 and HCM, showed a more similar morphology to adult CMs and led to changes in genes expression similar to during the maturation CMs in vivo (such as TNNT2, SERCA2, TNNI3, SCN5A) for cells grown on PCL nanofibers. In turn, cells isolated from the atrium, such as HL-1, showed a more anisotropic and rod-like shape for cultures grown on PU nanofibers; however, the level of gene expression changes most similar to mature CMs (such as TNNI3, ACTN2, MYL2) for cultures grown on PCL nanofibers. These results indicate that the changes at the genetic level responsible for maturation are more appropriate for cultures growing on PCL nanofibers. However, PU nanofibers also stimulate the mechanisms involved in CM maturation. This may be due to differences between cell types and biomaterial types. For example, ventricular cells require biomaterials that will be more elastic, such as PCL, while atrial cells need a more stiffness material, such as PU [67, 69]. Also, functional groups that differ depending on biomaterials may have different effects on atrial and ventricular cells because they differ in electrophysiology [69]. Even though numerous biomaterials with different physicochemical properties are commonly studied in the literature and are also compared with each other. However, to the best of our knowledge, there is no information on how the nanofibers with similar physical properties such as porosity, elasticity, fiber diameter but produced from other biomaterial affects various types of cardiac cells. The results of our article indicate interspecies differences in CM's biomaterial response. It can be noted that HCMs similarly to rat cardiomyoblasts exhibit greater orientation, morphology, and expression levels of the studied genes when cultured on nanofibers from PCL compared to cultures grown on PU nanofibers and PS. In contrast, mouse CMs cultured on PU nanofibers show the most similar morphology and physiology to adult CMs compared to cells grown on PCL nanofibers and PS. Additionally, different maturation mechanisms are triggered in human and rodent CMs. This correlation can be seen by comparing the results for protein and gene expression. From the results, rodent cells show a higher MYH6/MYH7 ratio cultured on nanofibers, and based on the literature sources is a rodent maturation mechanism in the cardiac tissue [41, 42]. Whereas HCMs increase in the MYH7/MYH6 ratio indicates more mature cells [41, 42]. In the case of gene expression, it can also determine the stimulation of different mechanisms for rodents and HCMs. It can be noted that the expression level of ACTN2 for HCMs changes slightly while there is a significant increase in the expression of this gene in rodent CMs. The expression of MYL2 on rodent and human cells is reversed depending on the biomaterial. The level of troponin T and troponin I is different depending on the species. For HCMs, the expression levels of TNNT2 and TNNI3 change significantly at later stages of CM development. In contrast, for rodents, this change mainly occurs during cardiomyoblast maturation and changes slightly during CM development. However, more research is needed in this direction to find nanofibrous mats with specific physicochemical properties which will be species-dependent and will be most similar to in vivo interactions. This kind of research may aid further studies into finding a suitable in vitro model of human cardiac tissue.
According to the above data, the selected nanofibers impact the morphological maturation of cardiac cells and affect the expression of proteins and genes involved in transforming fetal CMs into adult forms.
5. Conclusion
To summarize, this research demonstrated that polymer nanofibers affect cell viability and morphology and stimulate the expression of genes and proteins specific to the maturation of cardiac cells. Additionally, the study compared and evaluated the impact of physicochemical properties, such as biomaterial, elasticity, and diameter of nanofibers, on the functioning of cardiac cells, which differ in the stage of development, species, and origin from part of the heart (ventricular or atrium). Different maturation and cell morphology were noticed depending on the types of nanofibers and the types of cells used in the research. Rat cardiomyoblasts (H9c2), mouse CMs (HL-1), and HCMs were used in the experiments. The cells were cultured on the aligned PCL and PU nanofibrous mats for 24 h and 10 d. It was noticed that H9c2, HCM, and HL-1 cells cultured on nanofibers showed higher cell viability, elongation, anisotropic shape, parallel orientation, and maturation than cells cultured on polystyrene plates.
Based on the results, ventricular and atrial cells have a more mature morphology and physiology grown on nanofibers with a diameter close to 500 nm and elasticity above 40 MPa than for cultures grown on nanofibers with diameters close to 250 nm and elasticity around 30 MPa. The ventricular cells had a more mature morphology, and changes in the level of genes and protein expression were most similar to the in vivo conditions for cultures grown on PCL nanofiber were determined. Atrial cells showed a more mature morphology on PU nanofibers, but protein and gene expression were closer to in vivo systems for cultures grown on PCL nanofibers. The results may indicate that PCL nanofibers may be a biomaterial that enhances the maturation of various cardiac cell types more than PU nanofibers.
Currently, in vitro cell models use immature cardiac cells with different morphology and physiology than adult CMs. In turn, in vivo rat and mouse models are the most widely used research models to help understand how cardiac tissue works. They are not adequate models for research on finding an effective treatment method for human diseases because there are differences between species that are not fully explained and understood. It was proved that nanofibrous mats could be used to develop a cellular model that mimics cardiac tissue in vivo. The differences between the maturation of cardiac cells from different species were also shown. The work can support research on understanding and explaining discrepancies between the cellular mechanisms of rodents and human hearts and the selection of nanofibers that will effectively support the maturation of CMs.
Acknowledgments
This work is supported by National Science Centre within a frame of SONATA BIS 9 Program No. 2019/34/E/ST5/00381. The authors would like to thank Dominik Kolodziejek from Warsaw University of Technology, Chair of Medical Biotechnology, Faculty of Chemistry, for helping to produce holders for nanofibrous mats. The authors would like to thank Professor Paweł Dobrzyń from Nencki Institute of Experimental Biology PAS, for support in part of biological studies and the selection of materials for bioassays.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Author contributions
Conceptualization, Z I, and E J; formal analysis, E J, and E K; funding acquisition, E J; investigation, Z I, E K, A K, S K, I L, and M W; methodology, Z I, E K, and E J; project administration, E J; supervision, E J, and E K writing—original draft, Z I; writing—review and editing, M W, E K, and E J. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
There are no conflicts to declare.
Supplementary data (0.5 MB DOCX)