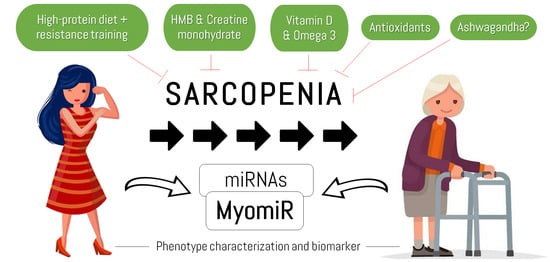
Sarcopenia: Etiology, Nutritional Approaches, and miRNAs
Abstract
:1. Introduction
2. Mechanisms
2.1. Muscle Protein Synthesis
2.2. Oxidative Stress
3. The Importance of MicroRNAs
3.1. Satellite Cell Regulation
3.2. Proteostasis
3.3. Size and Type of Muscle Fiber
3.4. ROS and Mitochondria
3.5. Fat Infiltration
3.6. miRNAs as a Therapy Tool and Biomarker
4. Counteracting Strategies
4.1. Physical Exercise with Emphasis on Resistance Training
4.2. Nutrition and Supplementation
4.2.1. High-Protein Diet
4.2.2. Nutritional Supplements
4.2.3. Antioxidants and Inflammation
5. Practical Nutritional Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/life-expectancy-and-healthy-life-expectancy (accessed on 24 May 2021).
- Hunter, G.R.; McCarthy, J.P.; Bamman, M.M. Effects of Resistance Training on Older Adults. Sports Med. 2004, 34, 329–348. [Google Scholar] [CrossRef]
- Latham, N.K.; Bennett, D.A.; Stretton, C.M.; Anderson, C.S. Systematic Review of Progressive Resistance Strength Training in Older Adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2004, 59, M48–M61. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Tufik, S.; de Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; Martínez-Rodríguez, A.; Gil-Arias, A. Impact of a motivational resistance-training programme on adherence and body composition in the elderly. Sci. Rep. 2018, 8, 1370. [Google Scholar] [CrossRef] [Green Version]
- Burgos Peláez, R. Enfoque terapéutico global de la sarcopenia. J. Nutr. Hosp. 2006, 21, 51–60. [Google Scholar]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Fleming, R.Y.D.; Wolfe, R.R. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J. Clin. Investig. 1995, 95, 811–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Aging 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Muscaritoli, M.; Anker, S.; Argiles, J.M.; Aversa, Z.; Bauer, J.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.T.; Malietzis, G.; Grove, T.N.; Jenkins, J.T.; Windsor, A.C.J.; Kontovounisios, C.; Warren, O. The emerging role of sarcopenia as a prognostic indicator in patients undergoing abdominal wall hernia repairs: A systematic review of the literature. Hernia 2020, 24, 1361–1370. [Google Scholar] [CrossRef]
- De Andrade, M.I.S.; Maio, R.; Dourado, K.F.; de Macêdo, P.F.C.; Neto, A.C.B. Excessive Weight—Muscle Depletion Paradox And Cardiovascular Risk Factors In Outpatients With Inflammatory Bowel Disease. Arq. Gastroenterol. 2015, 52, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shachar, S.S.; Williams, G.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Minis-Rutten, I.J.; Kruitwagen, R.F.; Kleijnen, J.; Lambrechts, S.; Damink, S.O.; Rensen, S.S.; Van Gorp, T. Sarcopenia and ovarian cancer survival: A systematic review and meta-analysis. J. Cachex-Sarcopenia Muscle 2019, 10, 1165–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strassmann, D.; Hensen, B.; Grünwald, V.; Stange, K.; Eggers, H.; Länger, F.; Omar, M.; Zardo, P.; Christiansen, H.; Reuter, C.W.; et al. Impact of sarcopenia in advanced and metastatic soft tissue sarcoma. Int. J. Clin. Oncol. 2021, 1–10. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachex-Sarcopenia Muscle 2017, 9, 3–19. [Google Scholar] [CrossRef]
- Vasilaki, A.; Richardson, A.; Van Remmen, H.; Brooks, S.; Larkin, L.; McArdle, A.; Jackson, M. Role of nerve-muscle interactions and reactive oxygen species in regulation of muscle proteostasis with ageing. J. Physiol. 2017, 595, 6409–6415. [Google Scholar] [CrossRef] [Green Version]
- Delbono, O. Neural control of aging skeletal muscle. Aging Cell 2003, 2, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, S.; Dyar, K.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachex-Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolomé, A.; García-Aguilar, A.; Asahara, S.-I.; Kido, Y.; Guillén, C.; Pajvani, U.B.; Benito, M. MTORC1 Regulates both General Autophagy and Mitophagy Induction after Oxidative Phosphorylation Uncoupling. Mol. Cell. Biol. 2017, 37, e00441-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinkovich, A.; Livshits, G. Sarcopenia—The search for emerging biomarkers. Ageing Res. Rev. 2015, 22, 58–71. [Google Scholar] [CrossRef]
- Crossland, H.; Kazi, A.A.; Lang, C.H.; Timmons, J.A.; Pierre, P.; Wilkinson, D.J.; Smith, K.; Szewczyk, N.; Atherton, P.J. Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1-associated pathway. Am. J. Physiol. Metab. 2013, 305, E183–E193. [Google Scholar] [CrossRef]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.; Milan, G.; Mammucari, C.; Meskers, C.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle. The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.D.; Phillips, S. Anabolic Processes in Human Skeletal Muscle: Restoring the Identities of Growth Hormone and Testosterone. Physician Sportsmed. 2010, 38, 97–104. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Gordon, S.E.; Fleck, S.J.; Marchitelli, L.J.; Mello, R.; Dziados, J.E.; Friedl, K.; Harman, E.; Maresh, C.; Fry, A.C. Endogenous Anabolic Hormonal and Growth Factor Responses to Heavy Resistance Exercise in Males and Females. Int. J. Sports Med. 1991, 12, 228–235. [Google Scholar] [CrossRef]
- Aoyama, S.; Shibata, S. The Role of Circadian Rhythms in Muscular and Osseous Physiology and Their Regulation by Nutrition and Exercise. Front. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, H.N.; Chen, C.C.W.; Hood, D.A. Mitochondria, Muscle Health, and Exercise with Advancing Age. Physiology 2015, 30, 208–223. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.; Wiggs, M.P.; et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J. Cachex-Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Mohamed, J.S.; Myers, M.J. Mitochondria Initiate and Regulate Sarcopenia. Exerc. Sport Sci. Rev. 2017, 45, 58–69. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Rimessi, A.; Previati, M.; Nigro, F.; Wieckowski, M.R.; Pinton, P. Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. Int. J. Biochem. Cell Biol. 2016, 81, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.P. Nutrition and Training Influences on the Regulation of Mitochondrial Adenosine Diphosphate Sensitivity and Bioenergetics. Sports Med. 2017, 47, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Milder, J.; Patel, M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2011, 100, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theurey, P.; Rieusset, J. Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol. Metab. 2016, 28, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Ojeda-Ojeda, M.; Murri, M.; Insenser, M.; Escobar-Morreale, H. Mediators of Low-Grade Chronic Inflammation in Polycystic Ovary Syndrome (PCOS). Curr. Pharm. Des. 2013, 19, 5775–5791. [Google Scholar] [CrossRef]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Byun, M.K.; Na Cho, E.; Chang, J.; Ahn, C.M.; Kim, H.J. Sarcopenia correlates with systemic inflammation in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 669–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle, S.; Koppo, K. Is inflammatory signaling involved in disease-related muscle wasting? Evidence from osteoarthritis, chronic obstructive pulmonary disease and type II diabetes. Exp. Gerontol. 2020, 137, 110964. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Quinlan, J.; Overthrow, K.; Greig, C.; Lord, J.; Armstrong, M.; Cooper, S. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients 2021, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Peng, P.; Shen, K. Role of Exosome Shuttle RNA in Cell-to-Cell Communication. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2016, 38, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Moran, Y.; Agron, M.; Praher, D.; Technau, U. The evolutionary origin of plant and animal microRNAs. Nat. Ecol. Evol. 2017, 1, 0027. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.N.; Kim, M.-S.; Adil, M.; Patil, A.H.; Lu, Y.; Mitchell, C.J.; Leal-Rojas, P.; Xu, J.; Kumar, M.; Dawson, V.L.; et al. Toward the human cellular microRNAome. Genome Res. 2017, 27, 1769–1781. [Google Scholar] [CrossRef] [Green Version]
- Kovanda, A.; Režen, T.; Rogelj, B. MicroRNA in skeletal muscle development, growth, atrophy, and disease. Wiley Interdiscip. Rev. RNA 2014, 5, 509–525. [Google Scholar] [CrossRef]
- McCarthy, J.J. The MyomiR Network in Skeletal Muscle Plasticity. Exerc. Sport Sci. Rev. 2011, 39, 150–154. [Google Scholar] [CrossRef]
- Soares, R.J.R.; Cagnin, S.; Chemello, F.; Silvestrin, M.; Musaro, A.; De Pitta, C.; Lanfranchi, G.; Sandri, M. Involvement of MicroRNAs in the Regulation of Muscle Wasting during Catabolic Conditions. J. Biol. Chem. 2014, 289, 21909–21925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchelson, K.R.; Qin, W.Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J Biol Chem. 2015, 6, 162–208. [Google Scholar] [CrossRef] [PubMed]
- Mytidou, C.; Koutsoulidou, A.; Katsioloudi, A.; Prokopi, M.; Kapnisis, K.; Michailidou, K.; Anayiotos, A.; Phylactou, L.A. Muscle-derived exosomes encapsulate myomiRs and are involved in local skeletal muscle tissue communication. FASEB J. 2021, 35, e21279. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Goljanek-Whysall, K. microRNAs: Modulators of the underlying pathophysiology of sarcopenia? Ageing Res. Rev. 2015, 24, 263–273. [Google Scholar] [CrossRef]
- Yin, J.; Qian, Z.; Chen, Y.; Li, Y.; Zhou, X. MicroRNA regulatory networks in the pathogenesis of sarcopenia. J. Cell. Mol. Med. 2020, 24, 4900–4912. [Google Scholar] [CrossRef]
- Chen, J.-F.; Tao, Y.; Li, J.; Deng, Z.; Yan, Z.; Xiao, X.; Wang, D.-Z. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J. Cell Biol. 2010, 190, 867–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, B.K.; Gagan, J.; Dutta, A. miR-206 and -486 Induce Myoblast Differentiation by Downregulating Pax7. Mol. Cell. Biol. 2011, 31, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Hirai, H.; Verma, M.; Watanabe, S.; Tastad, C.; Asakura, Y.; Asakura, A. MyoD regulates apoptosis of myoblasts through microRNA-mediated down-regulation of Pax3. J. Cell Biol. 2010, 191, 347–365. [Google Scholar] [CrossRef] [Green Version]
- Fochi, S.; Giuriato, G.; De Simone, T.; Gomez-Lira, M.; Tamburin, S.; Del Piccolo, L.; Schena, F.; Venturelli, M.; Romanelli, M. Regulation of microRNAs in Satellite Cell Renewal, Muscle Function, Sarcopenia and the Role of Exercise. Int. J. Mol. Sci. 2020, 21, 6732. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.; Paul, R.; Garros, R.F.; Natanek, S.A.; Bloch, S.; Lee, J.; Lorenzo, J.P.; Patel, H.; Cooper, C.; Sayer, A.A.; et al. miR-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting. J. Cachex-Sarcopenia Muscle 2017, 9, 400–416. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Vajjala, A.; Arigela, H.; Lokireddy, S.; Ge, X.; Bonala, S.; Manickam, R.; Kambadur, R.; Sharma, M. Negative Auto-Regulation of Myostatin Expression is Mediated by Smad3 and MicroRNA-27. PLoS ONE 2014, 9, e87687. [Google Scholar] [CrossRef] [Green Version]
- John, A.; Kubosumi, A.; Reddy, P.H. Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases. Cells 2020, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Rippo, M.R.; Olivieri, F.; Monsurrò, V.; Prattichizzo, F.; Albertini, M.C.; Procopio, A.D. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 2014, 56, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Bollheimer, L.C.; Buettner, R.; Pongratz, G.; Brunner-Ploss, R.; Hechtl, C.; Banas, M.; Singler, K.; Hamer, O.W.; Stroszczynski, C.; Sieber, C.C.; et al. Sarcopenia in the aging high-fat fed rat: A pilot study for modeling sarcopenic obesity in rodents. Biogerontology 2012, 13, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, L.; Hu, X.; Liu, L.; Xing, Y.; Zhou, Z.; Liang, X.; Yang, Q.; Jin, S.; Bao, J.; Gao, H.; et al. bta-miR-23a involves in adipogenesis of progenitor cells derived from fetal bovine skeletal muscle. Sci. Rep. 2017, 7, 43716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Li, Y.; Wang, X.-Y.; Zhang, D.; Zhang, H.; Wu, Q.; He, Y.-Q.; Wang, J.-Y.; Zhang, L.; Xia, H.; et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia 2013, 56, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-C.; Han, D.-S.; Hsu, C.-C.; Wang, J.-S. Circulating MicroRNA-486 and MicroRNA-146a serve as potential biomarkers of sarcopenia in the older adults. BMC Geriatr. 2021, 21, 86. [Google Scholar] [CrossRef]
- Gan, Z.; Fu, T.; Kelly, D.P.; Vega, R.B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018, 28, 969–980. [Google Scholar] [CrossRef]
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018, 10, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, L.A.; Sumukadas, D. Optimal management of sarcopenia. Clin. Interv. Aging 2010, 5, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamen, G.; Knight, C.A. Training-Related Adaptations in Motor Unit Discharge Rate in Young and Older Adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2004, 59, 1334–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, N.D.; Maganaris, C.N.; Narici, M.V. Effect of strength training on human patella tendon mechanical properties of older individuals. J. Physiol. 2003, 548, 971–981. [Google Scholar] [CrossRef]
- Chalé, A.; Cloutier, G.J.; Hau, C.; Phillips, E.M.; Dallal, G.E.; Fielding, R.A. Efficacy of Whey Protein Supplementation on Resistance Exercise–Induced Changes in Lean Mass, Muscle Strength, and Physical Function in Mobility-Limited Older Adults. Journals Gerontol. Ser. A Boil. Sci. Med. Sci. 2012, 68, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-J.; Latham, N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009, 2009, CD002759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, M.D.; Rhea, M.R.; Sen, A.; Gordon, P. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res. Rev. 2010, 9, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.A.; Quine, S.; Clemson, L.M.; Williams, E.J.; Williamson, D.A.; Stavrinos, T.M.; Grady, J.N.; Perry, T.J.; Lloyd, B.D.; Smith, E.; et al. Effects of High-Intensity Progressive Resistance Training and Targeted Multidisciplinary Treatment of Frailty on Mortality and Nursing Home Admissions after Hip Fracture: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2012, 13, 24–30. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Peterson, M.D.; Sen, A.; Gordon, P. Influence of Resistance Exercise on Lean Body Mass in Aging Adults: A meta-analysis. Med. Sci. Sports Exerc. 2011, 43, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkey, D.B.; Pollock, M.L.; Ishida, Y.; Welsch, M.A.; Brechue, W.F.; Graves, J.E.; Feigenbaum, M.S. Effect of resistance training volume on strength and muscle thickness. Med. Sci. Sports Exerc. 1996, 28, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Law, T.D.; Clark, L.A.; Clark, B.C. Resistance Exercise to Prevent and Manage Sarcopenia and Dynapenia. Annu. Rev. Gerontol. Geriatr. 2016, 36, 205–228. [Google Scholar] [CrossRef] [Green Version]
- Cannataro, R.; Di Maio, L.; Malorgio, A.; Micheli, M.L.; Cione, E. Spondyloarthritis and Strength Training: A 4-Year Report. J. Funct. Morphol. Kinesiol. 2021, 6, 58. [Google Scholar] [CrossRef]
- Malorgio, A.; Malorgio, M.; Benedetti, M.; Casarosa, S.; Cannataro, R. High intensity resistance training as intervention method to knee osteoarthritis. Sports Med. Health Sci. 2021, 3, 46–48. [Google Scholar] [CrossRef]
- Kakehi, S.; Wakabayashi, H.; Inuma, H.; Inose, T.; Shioya, M.; Aoyama, Y.; Hara, T.; Uchimura, K.; Tomita, K.; Okamoto, M.; et al. Rehabilitation Nutrition and Exercise Therapy for Sarcopenia. World J. Men’s Health 2021, 39, e13. [Google Scholar] [CrossRef]
- Wall, B.T.; van Loon, L.J. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013, 71, 195–208. [Google Scholar] [CrossRef]
- Barbiera, A.; Pelosi, L.; Sica, G.; Scicchitano, B.M. Nutrition and microRNAs: Novel Insights to Fight Sarcopenia. Antioxidants 2020, 9, 951. [Google Scholar] [CrossRef]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Essential Amino Acids Increase MicroRNA-499, -208b, and -23a and Downregulate Myostatin and Myocyte Enhancer Factor 2C mRNA Expression in Human Skeletal Muscle. J. Nutr. 2009, 139, 2279–2284. [Google Scholar] [CrossRef]
- Lançon, A.; Kaminski, J.; Tili, E.; Michaille, J.-J.; Latruffe, N. Control of MicroRNA Expression as a New Way for Resveratrol To Deliver Its Beneficial Effects. J. Agric. Food Chem. 2012, 60, 8783–8789. [Google Scholar] [CrossRef] [PubMed]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef] [Green Version]
- Canto, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Hector, A.J.; McGlory, C.; Damas, F.; Mazara, N.; Baker, S.K.; Phillips, S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2017, 32, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.H.; Churchward-Venne, T.A.; Mitchell, C.J.; Kolar, N.M.; Kassis, A.; Karagounis, L.G.; Burke, L.M.; Hawley, J.A.; Phillips, S.M. Hypoenergetic diet-induced reductions in myofibrillar protein synthesis are restored with resistance training and balanced daily protein ingestion in older men. Am. J. Physiol. Metab. 2015, 308, E734–E743. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, R.; Moura, E.G.; Dos Santos, V.C.; Caldeira, E.J.; Conte, M.; Matsumura, C.Y.; Pertille, A.; Mosqueira, M. High-fat diet suppresses the positive effect of creatine supplementation on skeletal muscle function by reducing protein expression of IGF-PI3K-AKT-mTOR pathway. PLoS ONE 2018, 13, e0199728. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, S.S.; Rensing, N.R.; Thio, L.L.; Yamada, K.A.; Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011, 52, e7–e11. [Google Scholar] [CrossRef] [Green Version]
- Paoli, A.; Bianco, A.; Damiani, E.; Bosco, G. Ketogenic Diet in Neuromuscular and Neurodegenerative Diseases. BioMed Res. Int. 2014, 2014, 474296. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Demontis, F. Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr. Opin. Pharmacol. 2017, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.R.; Scott, I.; Traba, J.; Han, K.; Sack, M.N. Regulation of autophagy and mitophagy by nutrient availability and acetylation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Smeuninx, B.; McKendry, J.; Wilson, D.; Martin, U.; Breen, L. Age-Related Anabolic Resistance of Myofibrillar Protein Synthesis Is Exacerbated in Obese Inactive Individuals. J. Clin. Endocrinol. Metab. 2017, 102, 3535–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beasley, J.M.; Shikany, J.M.; Thomson, C.A. The Role of Dietary Protein Intake in the Prevention of Sarcopenia of Aging. Nutr. Clin. Pract. 2013, 28, 684–690. [Google Scholar] [CrossRef] [Green Version]
- Courtney-Martin, G.; Ball, R.O.; Pencharz, P.B.; Elango, R. Protein Requirements during Aging. Nutrients 2016, 8, 492. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witard, O.C.; Wardle, S.L.; Macnaughton, L.S.; Hodgson, A.B.; Tipton, K.D. Protein Considerations for Optimising Skeletal Muscle Mass in Healthy Young and Older Adults. Nutrients 2016, 8, 181. [Google Scholar] [CrossRef]
- Kim, J.E.; O’Connor, L.E.; Sands, L.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, G.D.M.; Cade, J.; Grant, P.J.; Hardie, L.J. Nutrition and the circadian system. Br. J. Nutr. 2016, 116, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Latre, A.; Eckel-Mahan, K. Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health. Mol. Metab. 2016, 5, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [Green Version]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruocco, C.; Segala, A.; Valerio, A.; Nisoli, E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care 2020, 24, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Guillet, C. Fast digestive proteins and sarcopenia of aging. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 37–41. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Rasmussen, B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef]
- Moberg, M.; Apró, W.; Ekblom, B.; van Hall, G.; Holmberg, H.-C.; Blomstrand, E. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am. J. Physiol.-Cell Physiol. 2016, 310, C874–C884. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.; Herda, T.; Fry, A.; Cooper, M.; Andre, M.; Gallagher, P. Endocrine responses and acute mTOR pathway phosphorylation to resistance exercise with leucine and whey. Biol. Sport 2017, 34, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M. Nutritional Supplements in Support of Resistance Exercise to Counter Age-Related Sarcopenia. Adv. Nutr. 2015, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Cholewa, J.M.; Dardevet, D.; Lima-Soares, F.; de Araújo Pessôa, K.; Oliveira, P.H.; Dos Santos Pinho, J.R.; Nicastro, H.; Xia, Z.; Cabido, C.E.; Zanchi, N.E. Dietary proteins and amino acids in the control of the muscle mass during immobilization and aging: Role of the MPS response. Amino Acids 2017, 49, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bhalla, M.; De Jager, P.; Gilca, M. An Overview on Ashwagandha: A Rasayana (Rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 208–213. [Google Scholar] [CrossRef]
- Raut, A.; Rege, N.; Shirolkar, S.; Pandey, S.; Tadvi, F.; Solanki, P.; Vaidya, R.; Vaidya, A.; Kene, K. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J. Ayurveda Integr. Med. 2012, 3, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durg, S.; Bavage, S.; Shivaram, S.B. Withania somnifera (Indian ginseng) in diabetes mellitus: A systematic review and meta-analysis of scientific evidence from experimental research to clinical application. Phytother. Res. 2020, 34, 1041–1059. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Loke, W.; Foo, N.X.; Tan, W.J.; Chan, H.W.; Lim, D.Y.; Yeo, W.S. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother. Res. 2019, 34, 583–590. [Google Scholar] [CrossRef]
- Durg, S.; Dhadde, S.; Vandal, R.; Shivakumar, B.; Charan, C.S. W ithania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: A systematic review and meta-analysis. J. Pharm. Pharmacol. 2015, 67, 879–899. [Google Scholar] [CrossRef] [PubMed]
- HoleČek, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachex-Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; D’Introno, A.; Rubele, S.; Caliari, C.; Gattazzo, S.; Zoico, E.; Mazzali, G.; Fantin, F.; Zamboni, M. The Potential of β-Hydroxy-β-Methylbutyrate as a New Strategy for the Management of Sarcopenia and Sarcopenic Obesity. Drugs Aging 2017, 34, 833–840. [Google Scholar] [CrossRef]
- Bear, D.E.; Cruz-Jentoft, A.J.; Stout, J.R. β-hydroxy-β-methylbutyrate supplementation in older persons—An update. Curr. Opin. Clin. Nutr. Metab. Care 2020, 24, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-L.; Wu, J.; Zhu, L.; Chan, R.; Wang, X.; Huang, D.; Tang, N.; Woo, J. Peripheral Blood T Cell Gene Expression Responses to Exercise and HMB in Sarcopenia. Nutrients 2021, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zeng, L.; Deng, J.; Duan, Y.; Li, F. β-hydroxy-β-methylbutyrate (HMB) improves mitochondrial function in myocytes through pathways involving PPARβ/δ and CDK4. Nutrition 2018, 60, 217–226. [Google Scholar] [CrossRef]
- Manjarrez-Montes-De-Oca, R. El ß-hidroxi-ß-metilbutirato (HMB) como suplemento nutricional (II): Mecanismos de acción moleculares y celulares. Nutr. Hosp. 2015, 31, 597–605. [Google Scholar] [CrossRef]
- Figueiredo, V.C.; Cameron-Smith, D. Is carbohydrate needed to further stimulate muscle protein synthesis/hypertrophy following resistance exercise? J. Int. Soc. Sports Nutr. 2013, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Howarth, K.R.; Phillips, S.M.; MacDonald, M.J.; Richards, D.; Moreau, N.A.; Gibala, M.J. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J. Appl. Physiol. 2010, 109, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Candow, D.; Forbes, S.; Kirk, B.; Duque, G. Current Evidence and Possible Future Applications of Creatine Supplementation for Older Adults. Nutrients 2021, 13, 745. [Google Scholar] [CrossRef]
- Forbes, S.; Candow, D.; Ostojic, S.; Roberts, M.; Chilibeck, P. Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults. Nutrients 2021, 13, 1912. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, D.; Kreider, R.; Stout, J.; Forero, D.; Kerksick, C.; Roberts, M.; Rawson, E. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Kim, S.; Yoon, D.; Kim, J.; Sung, D.J. Role of creatine supplementation in exercise-induced muscle damage: A mini review. J. Exerc. Rehabil. 2015, 11, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.; Aagaard, P.; Kadi, F.; Tufekovic, G.; Verney, J.; Olesen, J.; Suetta, C.; Kjaer, M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J. Physiol. 2006, 573, 525–534. [Google Scholar] [CrossRef]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Hickner, R.; Ormsbee, M. The Potential Role of Creatine in Vascular Health. Nutrients 2021, 13, 857. [Google Scholar] [CrossRef]
- Solis, M.; Artioli, G.; Gualano, B. Potential of Creatine in Glucose Management and Diabetes. Nutrients 2021, 13, 570. [Google Scholar] [CrossRef]
- Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, P.-O. β-Hydroxy-β-methylbutyrate (HMB) and prevention of muscle wasting. Metabolism 2014, 63, 5–8. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2021, 79, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ticinesi, A.; Meschi, T.; Lauretani, F.; Felis, G.; Franchi, F.; Pedrolli, C.; Barichella, M.; Benati, G.; Di Nuzzo, S.; Ceda, G.P.; et al. Nutrition and Inflammation in Older Individuals: Focus on Vitamin D, n-3 Polyunsaturated Fatty Acids and Whey Proteins. Nutrients 2016, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- Halfon, M.; Phan, O.; Teta, D. Vitamin D: A Review on Its Effects on Muscle Strength, the Risk of Fall, and Frailty. BioMed Res. Int. 2015, 2015, 953241. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.; Costa, P.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Walsh, J.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Duque, G. Vitamin D in the aging musculoskeletal system: An authentic strength preserving hormone. Mol. Asp. Med. 2005, 26, 203–219. [Google Scholar] [CrossRef]
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The effect of vitamin D plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2021, 145, 56–63. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Kundu, J.K.; Na, H.-K. Nrf2 as a Master Redox Switch in Turning on the Cellular Signaling Involved in the Induction of Cytoprotective Genes by Some Chemopreventive Phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Cannataro, R.; Perri, M.; Gallelli, L.; Caroleo, M.C.; De Sarro, G.; Cione, E. Ketogenic Diet Acts on Body Remodeling and MicroRNAs Expression Profile. MicroRNA 2019, 8, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxidative Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, K.; Harper, W.; Bomser, J. Effect of whey protein isolate on intracellular glutathione and oxidant-induced cell death in human prostate epithelial cells. Toxicol. Vitr. 2002, 17, 27–33. [Google Scholar] [CrossRef]
- Liberman, K.; Njemini, R.; Luiking, Y.; Forti, L.N.; Verlaan, S.; Bauer, J.M.; Memelink, R.; Brandt, K.; Donini, L.; Maggio, M.; et al. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: The PROVIDE study. Aging Clin. Exp. Res. 2019, 31, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerksick, C.; Willoughby, D. The Antioxidant Role of Glutathione and N-Acetyl-Cysteine Supplements and Exercise-Induced Oxidative Stress. J. Int. Soc. Sports Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mealey, G.B.; Berry, W.; Plafker, S.M. Sulforaphane is a Nrf2-independent inhibitor of mitochondrial fission. Redox Biol. 2016, 11, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, C.; Finch, M.; Dunham, T.; Murphy, J.; Roy, B.; MacPherson, R. Creatine Monohydrate Supplementation Increases White Adipose Tissue Mitochondrial Markers in Male and Female Rats in a Depot Specific Manner. Nutrients 2021, 13, 2406. [Google Scholar] [CrossRef]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Wylie-Rosett, J.; Aebersold, K.; Conlon, B.; Isasi, C.R.; Ostrovsky, N.W. Health Effects of Low-Carbohydrate Diets: Where Should New Research Go? Curr. Diabetes Rep. 2012, 13, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Laeger, T.; Metges, C.C.; Kuhla, B. Role of β-hydroxybutyric acid in the central regulation of energy balance. Appetite 2010, 54, 450–455. [Google Scholar] [CrossRef]
- Ashcroft, S.P.; Bass, J.J.; Kazi, A.A.; Atherton, P.J.; Philp, A. The vitamin D receptor regulates mitochondrial function in C2C12 myoblasts. Am. J. Physiol. Physiol. 2020, 318, C536–C541. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; La Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic Diet and microRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Haces, M.L.; Hernández-Fonseca, K.; Medina-Campos, O.N.; Montiel, T.; Pedraza-Chaverri, J.; Massieu, L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp. Neurol. 2008, 211, 85–96. [Google Scholar] [CrossRef]
- Jarrett, S.; Milder, J.B.; Liang, L.-P.; Patel, M. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 2008, 106, 1044–1051. [Google Scholar] [CrossRef]
- Barry, D.; Ellul, S.; Watters, L.; Lee, D.; Haluska, R.; White, R. The ketogenic diet in disease and development. Int. J. Dev. Neurosci. 2018, 68, 53–58. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Hall, K.D.; Guo, J.; Ravussin, E.; Mayer, L.S.; Reitman, M.L.; Smith, S.R.; Walsh, B.T.; Leibel, R.L. Glucose and Lipid Homeostasis and Inflammation in Humans Following an Isocaloric Ketogenic Diet. Obesity 2019, 27, 971–981. [Google Scholar] [CrossRef]
- Fritsche, K.L. The Science of Fatty Acids and Inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstat, M.; Das, A. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA 2017, 114, E6034–E6043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, A.; Tingö, L.; Brummer, R. The Potential Effects of Probiotics and ω-3 Fatty Acids on Chronic Low-Grade Inflammation. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Arble, D.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Deutz, N.E.; Wolfe, R.R. Is there a maximal anabolic response to protein intake with a meal? Clin. Nutr. 2012, 32, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Bielohuby, M.; Fleming, T.; Grabner, G.; Foppen, E.; Bernhard, W.; Guzmán-Ruiz, M.; Layritz, C.; Legutko, B.; Zinser, E.; et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol. Metab. 2017, 6, 897–908. [Google Scholar] [CrossRef]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia—What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef] [Green Version]
- Cannataro, R.; Fazio, A.; La Torre, C.; Caroleo, M.; Cione, E. Polyphenols in the Mediterranean Diet: From Dietary Sources to microRNA Modulation. Antioxidants 2021, 10, 328. [Google Scholar] [CrossRef]
- Dev, P.K.; Gray, A.J.; Scott-Hamilton, J.; Hagstrom, A.D.; Murphy, A.; Denham, J. Co-expression analysis identifies networks of miRNAs implicated in biological ageing and modulated by short-term interval training. Mech. Ageing Dev. 2021, 199, 111552. [Google Scholar] [CrossRef]

| Nutrient | Rationale and Effects |
|---|---|
| Amino acids | The branched-chain amino acids (BCAAs), especially leucine, have been shown to play an important role in stimulating muscle protein synthesis through activation of the mTORC1 pathway [114,115]. Nevertheless, it seems that skeletal muscle depends on all essential amino acids rather than BCAAs or leucine alone [115,116,117]. In this sense, a growing body of evidence suggests that essential amino acids might extend healthy life span and prevent pathological conditions associated with an energy deficit (e.g., sarcopenia) [109]. These effects are possibly mediated through mitochondrial biogenesis and the upregulation of antioxidant systems [118]. |
| Ashwagandha | Withania somnifera (Ashawagandha), also called “Indian ginseng”, is an herb highly valued and used for centuries by Ayurvedic medicine, mainly for its adaptogenic and antistress activity, which is considered a proven Rasavātam [119] Due to its safety and effectiveness in improving quality of life and physical performance while decreasing fatigability [120], Ashwagandha is one of the most studied herbal products with potential antimicrobial, anti-inflammatory, antitumor, antistress, neuroprotective, cardioprotective, antioxidant, and antidiabetic properties [121,122,123]. |
| HMB | Clinical research has demonstrated the potential muscle protective effects of β-hydroxy-β-methylbutyrate (HMB), a leucine-derived molecule, in conditions that compromise skeletal muscle tissue [124,125,126]. These studies suggest that the p38/MAPK and PI3K/Akt signaling pathways are involved in the anticatabolic effects of HMB in skeletal muscle [123]. In addition to these mechanisms, possibly mechanisms involve inhibition of NF-κB activity, prevention of ROS production, stimulation of muscle cell proliferation and differentiation, inhibition of UPS, inhibition of caspases 3 and 8, and stabilization of the sarcolemma [127,128,129]. |
| Carbohydrates | Although there is no additional benefit of adding carbohydrates to a protein supplement that maximally stimulates muscle protein synthesis [130], muscle glycogen content may affect protein turnover [131]. Insulin is a powerful anabolic hormone that can stimulate not only protein synthesis but also amino-acid transport inside the cell [8]; therefore, sufficient carbohydrate intake may be beneficial for maintaining muscle mass in muscle-wasting conditions such as sarcopenia. |
| Creatine monohydrate | There are several studies showing that creatine monohydrate supplementation in addition to a strength training protocol can augment muscle mass and function in older adults [132,133,134]. It has been shown that this might be due to energy and mechanical optimization of the cells [135], which results in the prevention of protein degradation [134], an increase in and activation of satellite cells [136,137], and an increase in glycogen synthesis [138]. In addition, potential benefits of creatine outside of musculoskeletal tissue have been demonstrated in the brain, the heart, vascular health, immune system, diabetes, and cancer, among others [138,139,140,141]. Thus, creatine supplementation may have a potential benefit on sarcopenia [142,143,144]. |
| Vitamin D | The elderly population is particularly at risk of vitamin D deficiencies, as their ability to generate precursor molecules in the skin is reduced with the advancement of age [145,146], as well as in obese subjects, probably because it is trapped in the adipose tissue due to low-grade inflammation [147,148,149]. In fact, low levels of vitamin D have been shown to be related to loss of muscle mass, falls, and frailty [150]. Thus, current evidence supports vitamin D supplementation to improve muscle strength [151]. |
| 30 to 40 Years Old | 40 to 50 Years Old | 50 to 60 Years Old | 60 to 70 Years Old | |
|---|---|---|---|---|
| No caloric deficit | 2.0–2.3 g∙kg−1 FFM | 2.3–2.6 g∙kg−1 FFM | 2.6–2.9 g∙kg−1 FFM | 2.9–3.2 g∙kg−1 FFM |
| Caloric deficit | 2.4–2.8 g∙kg−1 FFM | 2.8–3.1 g∙kg−1 FFM | 3.1–3.5 g∙kg−1 FFM | 3.5–3.8 g∙kg−1 FFM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. https://doi.org/10.3390/ijms22189724
Cannataro R, Carbone L, Petro JL, Cione E, Vargas S, Angulo H, Forero DA, Odriozola-Martínez A, Kreider RB, Bonilla DA. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. International Journal of Molecular Sciences. 2021; 22(18):9724. https://doi.org/10.3390/ijms22189724
Chicago/Turabian StyleCannataro, Roberto, Leandro Carbone, Jorge L. Petro, Erika Cione, Salvador Vargas, Heidy Angulo, Diego A. Forero, Adrián Odriozola-Martínez, Richard B. Kreider, and Diego A. Bonilla. 2021. "Sarcopenia: Etiology, Nutritional Approaches, and miRNAs" International Journal of Molecular Sciences 22, no. 18: 9724. https://doi.org/10.3390/ijms22189724