Abstract
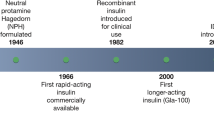
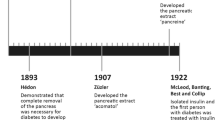
The basal-bolus concept of delivering insulin to diabetic patients makes physiological sense, as it mimics normal insulin release in people without diabetes. In line with this concept, a major effort put forth by insulin manufacturers has been to develop the ideal exogenous basal insulin product. The perfect basal insulin product would be injected into subcutaneous tissue without causing irritation, release insulin continuously at a constant rate for at least 24 hours, be stable, not contribute to weight gain, have a low risk of allergic reactions and, very importantly, minimize the risk of hypoglycaemia. While the perfect insulin has not yet been discovered, advancements are still being made.
Insulin degludec is an ultra-long-acting basal insulin analogue that possesses a flat, stable glucose-lowering effect in patients with type 1 or type 2 diabetes mellitus. Insulin degludec achieves these pharmacokinetic properties by forming soluble multihexamers upon subcutaneous injection, resulting in the formation of a depot in the subcutaneous tissue that is slowly released and absorbed into circulation. Insulin degludec has been associated with slightly less weight gain and fewer nocturnal hypoglycaemic episodes when compared with insulin glargine in some, but not all, clinical studies. This article briefly reviews current evidence for the use of insulin degludec in patients with type 1 or type 2 diabetes mellitus and discusses the potential impact of this new basal insulin on clinical practice.
Similar content being viewed by others
References
Keen H, Glynne A, Pickup JC, et al. Human insulin produced by recombinant DNA technology: safety and hypoglycaemic potency in healthy men. Lancet 1980; 2: 398–401
Jonassen I, Haelund S, Hoeg-Jensen, et al. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res 2012; 29(8): 2104–14
Kurtzhals P, Heise T, Strauss HM, et al. Multi-hexamer formation is the underlying mechanism behind the ultra-long glucose-lowering effect of insulin degludec. American Diabetes Association 71st Scientific Sessions; 2011 Jun 24–28; San Diego (CA)
Nosek L, Heise T, Bottcher SG, et al. Ultra-long-acting insulin degludec has a flat and stable glucose lowering effect [poster]. American Diabetes Association 71st Scientific Sessions; 2011 Jun 24–28; San Diego (CA)
Heise T, Hovelmann U, Nosek L, et al. Insulin degludec has a two-fold longer half-life and a more consistent pharmacokinetic profile than insulin glargine [poster]. American Diabetes Association 71st Scientific Sessions; 2011 Jun 24–28; San Diego (CA)
Zinman B, Fulcher G, Rao PV, et al. Insulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes: a 16-week, randomized, open-label, phase 2 trial. Lancet 2011; 377: 924–31
Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomized, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379: 1498–507
Birkeland KI, Home PD, Wendisch U, et al. Insulin degludec in type 1 diabetes. Diabetes Care 2011; 34: 661–5
Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomized, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379: 1489–97
Meneghini L, Atkin SL, Bain S, et al. Flexible once-daily dosing of insulin degludec does not compromise glycemic control or safety compared to insulin glargine given once daily at the same time each day in people with type 2 diabetes [abstract no. 35-LB; online]. Available from URL: http://professional.diabetes.org/Abstracts_Dis-play.aspx?TYP=1&CID=88737 [Accessed 2012 Nov 1]
Heise T, Tack CJ, Cuddihy R, et al. A new-generation ultra-long-acting basal insulin with a bolus boost compared with insulin glargine in insulin-naive people with type 2 diabetes. Diabetes Care 2011; 34: 669–74
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, J.D., Neumiller, J.J. & Campbell, R.K. Can a New Ultra-Long-Acting Insulin Analogue Improve Patient Care? Investigating the Potential Role of Insulin Degludec. Drugs 72, 2319–2325 (2012). https://doi.org/10.2165/11642240-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11642240-000000000-00000