Abstract
Edoxaban is an oral direct factor Xa inhibitor that is currently undergoing investigation in phase III clinical trials for the prevention of stroke in patients with atrial fibrillation (AF) and for the prevention and treatment of venous thromboembolic events (VTE).
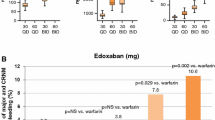
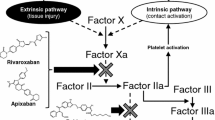
Factor Xa is an attractive target for anticoagulant treatment, as it is the primary and rate-limiting source of amplification in the coagulation cascade. Edoxaban is a competitive inhibitor of factor Xa and has >10 000-fold greater selectivity for factor Xa relative to thrombin. In phase I clinical trials, the anticoagulant effects of edoxaban included dose-dependent increases in activated partial thromboplastin time and prothrombin time following single edoxaban doses of 10–150 mg and after multiple ascending doses (60 mg twice daily, 90 mg daily and 120 mg daily). The anticoagulant effects of edoxaban were rapid in onset (time to peak plasma concentration 1–2 hours) and sustained for up to 24 hours. Prolongation of bleeding time in 8% of subjects was >9.5 minutes (none of which appeared to be clinically significant) 2 hours after initial dosing, and was independent of edoxaban dose, formulation or dietary state. In general, plasma edoxaban concentrations were linearly correlated with coagulation parameters. Phase II clinical trials in patients with AF and VTE suggest that the edoxaban 30 mg once-daily and 60 mg once-daily regimens had a similar or better safety profile compared with dose-adjusted warfarin (international normalized ratio 2.0-3.0) in terms of bleeding events, and that edoxaban was not associated with hepatotoxicity. In addition, edoxaban was associated with statistically significant dose-dependent reductions in VTE after orthopaedic surgery compared with placebo or dalteparin sodium. Further clinical investigation of the efficacy and safety of once-daily edoxaban is being conducted in phase III clinical trials in comparison with warfarin in patients with AF in the phase III ENGAGE AF-TIMI 48 trial (NCT00781391), and in comparison with low-molecular weight heparin/warfarin in the prevention of recurrent VTE in patients with symptomatic deep vein thrombosis and/or pulmonary embolism in the HOKUSAI VTE trial (NCT00986154).












Similar content being viewed by others
References
Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation — executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 2006 Aug 15; 48(4): 854–906
Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010 Oct; 31(19): 2369–429
Camm AJ, Kirchhof P, Lip GY, et al. on behalf of the European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010 Oct; 12(10): 1360–420
Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008 Jun; 133 (6 Suppl.): 381S–453S
Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008 Jun; 133 (6 Suppl.): 454S–545S
Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004 Sep; 126 (3 Suppl.): 204S–33S
Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008 Sep; 29(18): 2276–315
Stone WM, Tonnessen BH, Money SR. The new anticoagulants. Perspect Vasc Surg Endovasc Ther 2007 Sep; 19(3): 332–5
Donnan GA, Dewey HM, Chambers BR. Warfarin for atrial fibrillation: the end of an era? Lancet Neurol 2004 May; 3(5): 305–8
Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007 May 29; 115(21): 2689–96
Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008 Nov 11; 118(20): 2029–37
Birman-Deych E, Radford MJ, Nilasena DS, et al. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke 2006 Apr; 37(4): 1070–4
Ali S, Hong M, Antezano ES, et al. Evaluation and management of atrial fibrillation. Cardiovasc Hematol Disord Drug Targets 2006 Dec; 6(4): 233–44
Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol 2008 Feb; 31(2): 55–62
Huisman MV, Bounameaux H. Treating patients with venous thromboembolism: initial strategies and long-term secondary prevention. Semin Vasc Med 2005 Aug; 5(3): 276–84
Bajpai A, Savelieva I, Camm AJ. Treatment of atrial fibrillation. Br Med Bull 2008 Dec 1; 88(1): 75–94
Ederhy S, Meuleman C, Hammoudi N, et al. Preventing cerebrovascular accidents during atrial fibrillation. Presse Med 2005 Oct 22; 34(18): 1315–24
Turpie AG. Oral, direct factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases. Arterioscler Thromb Vasc Biol 2007 Jun; 27(6): 1238–47
Ahrens I, Lip GY, Peter K. New oral anticoagulant drugs in cardiovascular disease. Thromb Haemost 2010 Jul 5; 104(1): 49–60
Mavrakanas T, Bounameaux H. The potential role of new oral anticoagulants in the prevention and treatment of thromboembolism. Pharmacol Ther 2011 Apr; 130(1): 46–58
Piccini JP, Lopes RD, Mahaffey KW. Oral factor Xa inhibitors for the prevention of stroke in atrial fibrillation. Curr Opin Cardiol 2010 Jul; 25(4): 312–20
Turpie AG. New oral anticoagulants in atrial fibrillation. Eur Heart J 2008 Jan; 29(2): 155–65
Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmaco-dynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet 2009; 48(1): 1–22
Weitz JI, Connolly SJ, Patel I, et al. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost 2010 Aug 5; 104(3): 633–41
Fuji T, Fujita S, Tachibana S, et al. A dose-ranging study evaluating the oral factor Xa inhibitor edoxaban for the prevention of venous thromboembolism in patients undergoing total knee arthroplasty. J Thromb Haemost 2010 Nov; 8(11): 2458–68
Hylek E. DU-176b, an oral, direct Factor Xa antagonist. Curr Opin Investig Drugs 2007 Sep; 8(9): 778–83
Lassen MR, Davidson BL, Gallus A, et al. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost 2007 Dec; 5(12): 2368–75
Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010 Mar 6; 375(9717): 807–15
Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009 Aug 6; 361(6): 594–604
Eriksson BI, Borris LC, Dahl OE, et al. A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation 2006 Nov 28; 114(22): 2374–81
Turpie AG, Fisher WD, Bauer KA, et al. BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement: a phase II dose-ranging study. J Thromb Haemost 2005 Nov; 3(11): 2479–86
ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J 2010 Mar; 159(3): 340–7 e1
Hacke W, Patel MR, Becker RC, et al. Baseline characteristics of the ROCKET AF study: comparison with recent atrial fibrillation studies [abstract OAID 5]. Poster presented at the 19th European Stroke Conference (ESC); 2010 May 25–8; Barcelona, Spain
Patel MR. Stroke prevention using the oral direct factor Xa Inhibitor rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation (ROCKET AF) [abstract 21839]. Circulation 2010; 122(21): 2215–26
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009 Sep 17; 361(12): 1139–51
Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007 Nov 1; 100(9): 1419–26
Furugohri T, Isobe K, Honda Y, et al. Pharmacological characterization, antithrombotic and bleeding effects of DU-176b, a novel, potent and orally active direct inhibitor of factor Xa: a wider safety margin of antithrombotic and bleeding effects compared to heparin, LMWH and warfarin [abstract]. J Thromb Haemost 2005; 3 Suppl. 1: P1 110
Ogata K, Mendell-Harary J, Tachibana M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol 2010 Jul; 50(7): 743–53
Zafar MU, Vorchheimer DA, Gaztanaga J, et al. Antithrombotic effects of factor Xa inhibition with DU-176b: phase-I study of an oral, direct factor Xa inhibitor using an ex-vivo flow chamber. Thromb Haemost 2007 Oct; 98(4): 883–8
Mendell J, Tachibana M, Shi M, et al. Effects of food on the pharmacokinetics of edoxaban, an oral direct factor Xa Inhibitor, in healthy volunteers. J Clin Pharmacol 2011 May; 51(5): 687–94
Kubitza D, Becka M, Zuehlsdorf M, et al. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59-7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol 2006 May; 46(5): 549–58
Stangier J, Eriksson BI, Dahl OE, et al. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol 2005 May; 45(5): 555–63
Ruff CT, Giugliano RP, Antman EM, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI48). Am Heart J 2010 Oct; 160(4): 635–41 e2
European Medicines Agency. Xarelto (rivaroxaban): summary of product characteristics. Bayer Schering Pharma; 2009 May
Walenga JM, Adiguzel C. Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract 2010 Jun; 64(7): 956–67
Pradaxa (dabigatran etexilate mesylate): US prescribing information. Ridgefield (CT): Boehringer Ingelheim Pharmaceuticals Inc, 2011 Ma
Masumoto H, Yoshigae Y, Watanabe K, et al. In vitro metabolism of edoxaban and the enzymes involved in the oxidative metabolism of edoxaban [abstract]. AAPS J 2010; 12(S2): W4308
Daiichi Sankyo Inc. Global study to assess the safety and effectiveness of DU-176b vs standard practice of dosing with warfarin in patients with atrial fibrillation (EngageAFTIMI48) [ClinicalTrials.gov identifier NCT00781391]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2011 Jul 13]
Furugohri T, Isobe K, Honda Y, et al. DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost 2008 Sep; 6(9): 1542–9
Morishima Y, Fukuda T, Honda Y, et al. Activated prothrombin complex concentrate, recombinant factor VIII and factor IX reverse prolonged clotting time induced by DU-176B, a direct factor XA inhibitor, in human plasma [abstract]. J Thromb Haemost 2007; 5 Suppl. 2: P–T–639
Morishima Y, Hornick P, Matsumoto C, et al. Recombinant factor VIIa reverses prolonged bleeding time induced by a high dose of DU-176b, a novel direct factor Xa inhibitor, in rats [abstract]. J Thromb Haemost 2005; 3 Suppl. 1 P0512
Morishima Y, Honda Y, Matsumoto C, et al. Thrombin generation is a possible marker for a haemostatic action of recombinant factor VIIa to shorten prolonged bleeding time provoked by a high dose of a direct factor Xa inhibitor in rats [abstract]. Eur Heart J 2008; 29 Suppl.: 331
Samama M, Mendell J, Guinet C, et al. Comparison of the effect of edoxaban and fondaparinux on the inhibition of thrombin generation (in-vitro study) [abstract]. Pathophysiol Haemost Thromb 2010; 37 Suppl. 1: P407
Samama M, Woltz M, Ogata K, et al. Effect of edoxaban (DU-176b) on thrombin generation and platelet activation in shed and venous blood with fondaparinux as active comparator [abstract]. ESC Congress 2009: Annual Congress of the European Society of Cardiology; 2009 Aug 29–Sep 2; Barcelona, Spain, P2105
Morishima Y, Shirasaki Y, Kito F, et al. A wide safety margin of a factor Xa inhibitor, DU-176b, between anti-thrombotic effect and exacerbation of intracerebral hemorrhage in rats: comparison with a thrombin inhibitor [abstract]. Circulation 2006; 114(II): 104
Mendell J, Basavapathruni R, Swearingen D, et al. A thorough electrocardiogram study of edoxaban, a novel factor Xa inhibitor. J Clin Pharmacol Epub 2011 Jan 5
Raskob G, Cohen AT, Eriksson BI, et al. Oral direct factor Xa inhibition with edoxaban for thromboprophylaxis after elective total hip replacement: a randomised double-blind dose-response study. Thromb Haemost 2010 Jun 29; 104(3): 642–9
Fuji T, Wang C-J, Fujita S, et al. Edoxaban in patients undergoing total hip arthroplasty: a phase IIb dose-finding study [abstract]. Blood 2009; 114: 2098
Naccarelli GV, Varker H, Lin J, et al. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009 Dec 1; 104(11): 1534–9
Anon. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994 Jul 11; 154 (13): 1449–57
Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002 Oct 1; 113(5): 359–64
Kirchhof P, Auricchio A, Bax J, et al. Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J 2007 Nov; 28(22): 2803–17
Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 2003 Sep 11; 349(11): 1019–26
Chung N, Jeon HK, Lien LM, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non-valvular atrial fibrillation. Thromb Haemost 2011 Mar; 105(3): 535–44
Yasaka M, Inoue H, Kawai Y, et al. Randomized, parallel group, warfarin control, multicenter phase II study evaluating safety of DU-176b in Japanese subjects with non-valvular atrial fibrillation (NVAF) [abstract]. J Thromb Haemost 2009 Jul; 7 Suppl. 2: PP–WE–196
Koretsune Y, Inoue H, Kawai Y, et al. The oral factor Xa inhibitor DU-176b in Japanese warfarin-naive patients with atrial fibrillation: results of two phase II open-label, dose-escalation studies [abstract]. 58th Annual Scientific Session of the American College of Cardiology; 2009 Mar 29–31; Orlando (FL), 1022-114
Mahaffey KW, Fox KA. Rivaroxaban once daily, oral, direct Factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Presented at the AHA Scientific Sessions; 2010 Nov 13–17; Chicago [online]. Available from URL: http://sciencenews.myamericanheart.org/pdfs/ROCKET_AF_pslides.pdf [Accessed 2011 Jan 17]
Eikelboom JW, O’Donnell M, Yusuf S, et al. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J 2010 Mar; 159(3): 348–53 e1
Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011 Mar 3; 364(9): 806–17
Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010 Dec 23; 363(26): 2499–510
Gibson CM, Mega JL, Burton P, et al. Rationale and design of the anti-Xa therapy to lower cardiovascular events in addition to standard therapy in subjects with acute coronary syndrome-thrombolysis in myocardial infarction 51 (ATLAS-ACS 2 TIMI 51) trial: a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of rivaroxaban in subjects with acute coronary syndrome. Am Heart J 2011 May; 161(5): 815–21
BusinessWire. APPRAISE-2 study with investigational compound apixaban in acute coronary syndrome discontinued. BusinessWire 2010 Nov 18 [online]. Available from URL: http://www.businesswire.com/news/bms/20101118007161/en/APPRAISE-2-Study-Investigational-Compound-Apixaban-Acute-Coronary [Accessed 2011 Feb 3]
Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009 Dec 10; 361(24): 2342–52
Buller HR, Lensing AW, Prins MH, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT dose-ranging study. Blood 2008 Sep 15; 112(6): 2242–7
Lopes RD, Alexander JH, Al-Khatib SM, et al. Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J 2010 Mar; 159(3): 331–9
Roskell NS, Lip GY, Noack H, et al. Treatments for stroke prevention in atrial fibrillation: a network meta-analysis and indirect comparisons versus dabigatran etexilate. Thromb Haemost 2010 Dec; 104(6): 1106–15
Daiichi-Sankyo. Study ID DU176b-D-U305 [data on file]
Daiichi Sankyo Inc. Comparative investigation of low molecular weight (LMW) heparin/edoxaban tosylate (DU176b) versus (LMW) heparin/warfarin in the treatment of symptomatic deep-vein blood clots and/or lung blood clots. (The Edoxaban Hokusai-VTE Study) [ClinicalTrials.gov identifier NCT00986154]. US National Institutes of Health, Clinical-Trials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2011 Jul 13]
Fuji T, Wang C-J, Fujita S, et al. Edoxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial [abstract]. Pathophysiol Haemost Thromb 2010; 37 Suppl. 1: OC297
Fujita S, Fuji T, Tachibana S, et al. Safety and efficacy of edoxaban in patients undergoing hip fracture surgery [abstract]. Pathophysiol Haemost Thromb 2010; 37 Suppl. 1: P366
Fuji T, Fujita S, Tachibana S, et al. Efficacy and safety of edoxaban versus enoxaparin for the prevention of venous thromboembolism following total hip arthroplasty: STARS J-V trial. ASH Annual Meeting Abstracts 2010 Nov19; 116(21): 3320
Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008 Jun 26; 358(26): 2765–75
Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008 Jul 5; 372(9632): 31–9
Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008 Jun 26; 358(26): 2776–86
Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009 May 16; 373(9676): 1673–80
Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007 Sep 15; 370(9591): 949–56
Acknowledgements
Medical writing and editing support funded by Daiichi Sankyo Europe GmbH was provided by Simon Lancaster and Ray Hill, from inScience Communications, a Wolters Kluwer business.
Professor Camm has received research grants from Pfizer, BMS, Daiichi Sankyo, St Jude, Servier and Sanofi-aventis, and honoraria for Speakers Bureaus and Advisory Boards from Cardiome, Sanofi-aventis, Menarini, Daiichi Sankyo, Pfizer, Merck, BMS, ARYx, Xention, Servier, Novartis, Actelion, Medtronic and Boston Scientific. Professor Bounameaux discloses having received a research grant from Sanofi-aventis, and honoraria for consulting/lecturing from Bayer-Schering Pharma, Boehringer-Ingelheim, BMS, GSK, Sanofi-aventis, Servier, Daiichi-Sankyo, Canonpharma and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Camm, A.J., Bounameaux, H. Edoxaban. Drugs 71, 1503–1526 (2011). https://doi.org/10.2165/11595540-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11595540-000000000-00000