Abstract
Background: The use of drugs for migraine during pregnancy may have adverse effects on delivery outcome, and warnings exist for such drugs regarding use during pregnancy. Most information in the literature concerns triptans.
Objective: The aim of the study was to describe the delivery outcome when a woman had used drugs for migraine during pregnancy.
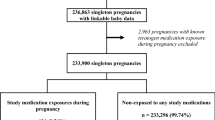
Study Design: A register study where exposure for drugs was obtained partly by interview conducted by the attending antenatal care midwife and medical records from antenatal care (1995–2008) and partly by linkage to the Prescribed Drug Register (2005–8).
Setting: All deliveries in Sweden (1 211 670 women) recorded in the Medical Birth Register with data from antenatal care.
Patients: Women using triptans or ergots during pregnancy were identified and compared with all women who did not use drugs for migraine.
Main Outcome Measures: Pregnancy complications, pregnancy duration and birthweight, neonatal morbidity and mortality, and congenital malformations.
Results: Use of ergots or triptans during early pregnancy (first trimester) occurred in 3286 women with 3327 infants, while use after the first trimester occurred in 1394 women with 1419 infants. Women using such drugs for migraine were older than other women, were more often of parity 1 (no previous infant) and more often had a high body mass index. Women using drugs for migraine had not previously had more miscarriages than expected. There was an increased risk for pre-eclampsia (odds ratio [OR] 1.44; 95% CI 1.17, 1.76). An increased risk for preterm birth was seen after use of drugs for migraine later in pregnancy (OR 1.50; 95% CI 1.22, 1.84). There was no increased risk for stillbirth or early neonatal death. No certain signs of teratogenicity were found for any of the drug types when compared with women not using such drugs (OR for any malformation 0.95; 95% CI 0.80, 1.12).
Conclusions: Our data suggest that the risk of adverse effects on pregnancy outcome associated with the use of drugs for migraine is low but data for triptans other than sumatriptan are still few.










Similar content being viewed by others
References
Pfaffenrath V, Rehm M. Migraine in pregnancy: what are the safest treatment options? Drug Saf 1998; 19: 383–8
Fox AW, Chambers CD, Anderson PO, et al. Evidence-based assessment of pregnancy outcome after sumatriptan exposure. Headache 2002; 42: 8–15
Fox AW. Revised estimate for probability of successful outcome of pregnancy after sumatriptan exposure [letter]. Headache 2004; 44: 842–3
Cunnington M, Ephross S, Churchill P. The safety of sumatriptan and naratriptan in pregnancy: what have we learned? Headache 2009; 49: 1414–22
Nezvalová-Henriksen K, Spigset O, Nordeng H. Triptan exposure during pregnancy and the risk of major congenital malformations and adverse pregnancy outcomes: results from the Norwegian Mother and Child Cohort Study. Headache 2010; 50: 563–75
Heinonen OP, Sloane D, Shapiro A. Birth defects and drugs in pregnancy. Littleton (MA): Publishing Sciences Group, Inc., 1977
Källén B, Lygner P-E. Delivery outcome in women who used drugs for migraine during pregnancy with special reference to sumatriptan. Headache 2001; 41: 351–6
Bánhidy F, Puhó E, Czeizel AE. A possible dose-dependent teratogenic effect of ergotamine [letter]. Reprod Toxicol 2006; 22: 551–2
Bánhidy F, Árcs N, Horváth-Puhó E, et al. Maternal severe migraine and risk of congenital limb deficiencies. Birth Defects Res Part A Clin Mol Teratol 2006; 76: 592–601
Bánhidy F, Árcs N, Horváth-Puhó E, et al. Pregnancy complications and delivery outcomes in pregnant women with severe migraine. Eur J Obstet Gynecol Reprod Biol 2006; 134: 157–63
Bánhidy F, Ács N, Puhó E, et al. Ergotamine treatment during pregnancy and a higher rate of low birth weight and preterm birth. Br J Pharmacol 2007; 64: 510–6
Graham Jr JM, Marin-Madilla M, Hoefnagel D. Jejunal atresia associated with Cafergot ingestion during pregnancy. Clin Pediatr (Phila) 1983; 22: 226–8
Barkovitch AJ, Rowley H, Bollen A. Correlation of prenatal events with the development of polymicrogyria. AJNR Am J Neuroradiol 1995; 16: 822–7
Smets K, Zecic A, Willems J. Ergotamine as a possible cause of Möbius sequence: additional clinical observation. J Child Neurol 2004; 19: 398
Källén B. Drugs during pregnancy. New York: Nova Science Publishers, 2009
National Board of Health and Welfare, Centre for Epidemiology. The Swedish Medical Birth Register: a summary of content and quality [online]. Available from URL: http://www.socialstyrelsen.se/Publikationer2003/2003-112-3 [Accessed 2010 May 12]
Källén B, Otterblad Olausson P. Monitoring of maternal drug use and infant congenital malformations: does loratadine cause hypospadias? Int J Risk Safety Med 2001; 14: 115–9
Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register: opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–35
National Board of Health and Welfare, Centre for Epidemiology. Registration of congenital malformations in Swedish health registers [online]. Available from URL: http://www.socialstyrelsen.se/Publikationer2004/2004-112-1 [Accessed 2010 May 12]
Källén B. A birth weight for gestational age standard based on data in the Swedish medical birth registry 1985–1989. Eur J Epidemiol 1995; 11: 601–6
Torelli P, Allais G, Marizoni GG. Clinical review of headache in pregnancy. Neurol Sci 2010; 31 Suppl. 1: S55-8
Tietjen GE, Herial NA, Hardgrove J, et al. Migraine comorbidity constellations. Headache 2007; 47: 857–65
Acknowledgement
This study was supported by a grant to Bengt Källén from the Evy and Gunnar Sandberg Foundation, Sweden. The authors’ work was independent of the funders. None of the authors declared a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Källén, B., Nilsson, E. & Olausson, P.O. Delivery Outcome after Maternal Use of Drugs for Migraine. Drug-Safety 34, 691–703 (2011). https://doi.org/10.2165/11590370-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11590370-000000000-00000