Abstract
Background
The pharmacoeconomics of the myeloid growth factors (MGFs) is an important topic that has received substantial attention in recent years. The use of the MGFs as primary prophylaxis to prevent febrile neutropenia (FN) has grown considerably over the past decade and professional guidelines regarding their use have broadened the settings in which these agents are indicated. Recent data also suggest a potential role for them in reducing infection-related and all-cause mortality. The cost and effectiveness of these agents will continue to gain visibility as companies pursue approval for bio-similar agents in the US, similar to their recent approval in Europe.
Objectives
The objective of this paper is to review the available pharmaco-economic literature on the MGFs, which is particularly timely in light of the recent passage of healthcare reform and the increasing focus on cost control. The cost of treating cancer in the US is rising faster than the already rapid increase in overall medical expenditure. The clinical utility and cost effectiveness of supportive care measures in oncology must therefore be weighed carefully. This review focuses on the use of different formulations of MGFs for primary and secondary prophylaxis of chemotherapy-induced neutropenia.
Methods
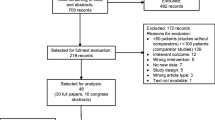
A MEDLINE search was performed to find studies that became available since the prior review of this topic was published in Pharmacoeconomics in 2003.
Results
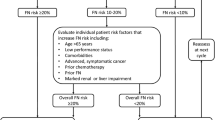
Acceptable cost-minimization estimates for primary prophylaxis with the MGFs in patients receiving cancer chemotherapy have been provided by several studies in the US. Of the commonly used agents in the US, pegfilgrastim appears to be superior to the currently recommended dose and schedule of filgrastim in terms of cost minimization, and primary prophylaxis appears to be less costly than secondary prophylaxis. However, the cost benefits of primary prophylaxis in Europe are not as pronounced as in the US, due to the lower costs of medical care. Data continue to emerge suggesting a decreased risk of early mortality from averted infections as well as the possibility of a disease-specific mortality benefit through maintaining the relative dose intensity of chemotherapy with MGF support.
Conclusion
This evidence will prove valuable in assessing the overall cost effectiveness and cost utility of the MGFs in patients receiving cancer chemotherapy.




Similar content being viewed by others
References
Caggiano V, Weiss RV, Rickert TS, et al. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 2005; 103 (9): 1916–24
Crawford J, Armitage J, Blayney DW, et al. Myeloid growth factors. J Natl Compr Canc Netw 2011; 9 (8): 914–31
Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006; 106 (10): 2258–66
Lyman GH, Michels SL, Reynolds MW, et al. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 2010; 116 (23): 5555–63
Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 2007; 25 (21): 3158–67
Ozer H, Armitage JO, Bennett CL, et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol 2000; 18 (20): 3558–85
American Society of Clinical Oncology. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol 1996; 14 (2): 671–9
Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 2011; 47: 8–32
Lyman GH. Guidelines of the National Comprehensive Cancer Network on the use of myeloid growth factors with cancer chemotherapy: a review of the evidence. J Natl Compr Canc Netw 2005. 3 (4): 557–71
Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 2006; 24 (19): 3187–205
National Cancer Institute. Cancer trends progress report: 2009/2010 update. Bethesda (MD): NCI, NIH, DCCPS, 2010 Apr [online]. Available from URL: http://progressreport.cancer.gov [Accessed 2011 Jan 10]
American Cancer Society. Cancer facts and figures. Atlanta (GA): American Cancer Society, 2009
Esser M, Brunner H. Economic evaluations of granulocyte colony-stimulating factor: in the prevention and treatment of chemotherapy-induced neutropenia. Pharmacoeconomics 2003; 21 (18): 1295–313
Uyl-de Groot CA, Huijgens PC, Rutten FFH. Colony-stimulating factors and peripheral blood progenitor cell transplantation: benefits and costs. Pharmacoeconomics 1996; 10(1): 23–35
Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 1991; 325 (3): 164–70
Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 2005; 23 (6): 1178–84
Timmer-Bonte JN, de Boo TM, Smit HJ, et al. Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch randomized phase III study. J Clin Oncol 2005; 23 (31): 7974–84
Cosler LE, Eldar-Lissai A, Culakova E, et al. Therapeutic use of granulocyte colony-stimulating factors for established febrile neutropenia: effect on costs from a hospital perspective. Pharmacoeconomics 2007; 25 (4): 343–51
Pettengell R, Gurney H, Radford JA, et al. Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin’s lymphoma: a randomized controlled trial. Blood 1992; 80 (6): 1430–14
Trillet-Lenoir V, Green J, Manegold C, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer 1993; 29A (3): 319–24
Zinzani PL, Pavone E, Storti S, et al. Randomized trial with or without granulocyte colony-stimulating factor as adjunct to induction VNCOP-B treatment of elderly high-grade non-Hodgkin’s lymphoma. Blood 1997; 89 (11): 3974–9
Fossa SD, Kaye SB, Mead GM, et al. Filgrastim during combination chemotherapy of patients with poor-prognosis metastatic germ cell malignancy: European Organization for Research and Treatment of Cancer, Genito-Urinary Group, and the Medical Research Council Testicular Cancer Working Party, Cambridge, United Kingdom. J Clin Oncol 1998; 16 (2): 716–24
Doorduijn JK, van der Holt B, van Imhoff GW, et al. CHOP compared with CHOP plus granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin’s lymphoma. J Clin Oncol 2003; 21 (16): 3041–50
Ösby E, Hagberg H, Kvaloy S, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood 2003; 101 (10): 3840–8
Gebbia V, Testa A, Valenza R, et al. A prospective evaluation of the activity of human granulocyte-colony stimulating factor on the prevention of chemotherapy-related neutropenia in patients with advanced carcinoma. J Chemother 1993; 5 (3): 186–90
Gebbia V, Valenza R, Testa A, et al. A prospective randomized trial of thymopentin versus granulocyte-colony stimulating factor with or without thymopentin in the prevention of febrile episodes in cancer patients undergoing highly cytotoxic chemotherapy. Anticancer Res 1994; 14 (28): 731–4
Chevallier B, Chollet P, Merrouche Y, et al. Lenograstim prevents morbidity from intensive induction chemotherapy in the treatment of inflammatory breast cancer. J Clin Oncol 1995; 13(7): 1564–71
Bui BN, Chevallier B, Chevreau C, et al. Efficacy of lenograstim on hematologic tolerance to MAID chemotherapy in patients with advanced soft tissue sarcoma and consequences on treatment dose-intensity. J Clin Oncol 1995; 13 (10): 2629–36
Gisselbrecht C, Haioun C, Lepage E, et al. Placebo-controlled phase III study of lenograstim (glycosylated re-combinant human granulocyte colony-stimulating factor) in aggressive non-Hodgkin’s lymphoma: factors influencing chemotherapy administration. Groupe d’Etude des Lymphomes de l’Adulte. Leuk Lymphoma 1997; 25 (3–4): 289–300
Talcott JA, Siegel RD, Finberg R, et al. Risk assessment in cancer patients with fever and neutropenia: a prospective, two-center validation of a prediction rule. J Clin Oncol 1992; 10(2): 316–22
Klastersky J, Paesmans JK, Rubenstein EB, et al. The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 2000; 18 (16): 3038–51
Gonzalez-Barca E, Fernández-Sevilla A, Carratalá J, et al. Prognostic factors influencing mortality in cancer patients with neutropenia and bacteremia. Eur J Clin Microbiol Infect Dis 1999; 18 (8): 539–44
Malik I, Hussain M, Yousuf H. Clinical characteristics and therapeutic outcome of patients with febrile neutropenia who present in shock: need for better strategies. J Infect 2001; 42 (2): 120–5
Elting LS, Rubenstein EB, Rolston KVI, et al. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis 1997; 25 (2): 247–59
Carratala J, Rosón B, Fernández-Sevilla A, et al. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch Intern Med 1998; 158 (8): 868–72
Bohlius J, Herbst C, Reiser M, et al. Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma. Cochrane Database Syst Rev 2008; 4: CD003189
Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol 2010; 28 (17): 2914–24
Lyman GH, Kleiner JM. Summary and comparison of myeloid growth factor guidelines in patients receiving cancer chemotherapy. J Natl Compr Canc Netw 2007; 5 (2): 217–28
Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011; 103(2): 117–28
Greenberg D, Earle C, Fang CH, et al. When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology. J Natl Cancer Inst 2010; 102 (2): 82–8
Pinto L, Liu Z, Doan Q, et al. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin 2007; 23 (9): 2283–95
Morrison VA, Wong M, Hershman D, et al. Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3–4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm 2007; 13 (4): 337–48
Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 2003; 14 (1): 29–35
Siena S, Picartt MJ, Holmes FA, et al. A combined analysis of two pivotal randomized trials of a single dose of pegfilgrastim per chemotherapy cycle and daily filgrastim in patients with stage II–IV breast cancer. Oncol Rep 2003; 10 (3): 715–24
Uys A, Rapoport BL, Anderson R. Febrile neutropenia: a prospective study to validate the Multinational Association of Supportive Care of Cancer (MASCC) risk-index score. Support Care Cancer 2004; 12 (8): 555–60
Cosler LE, Lyman GH. Economic analysis of gene expression profile data to guide adjuvant treatment in women with early-stage breast cancer. Cancer Invest 2009; 27 (10): 953–9
Anderson GF, Frogner BK. Health spending in OECD countries: obtaining value per dollar. Health Aff (Millwood) 2008; 27 (6): 1718–27
Schoen C, Osborn R, Squires D, et al. How health insurance design affects access to care and costs, by income, in eleven countries. Health Aff (Millwood) 2010; 29 (12): 2323–34
Lyman GH, Kuderer N, Greene J, et al. The economics of febrile neutropenia: implications for the use of colony-stimulating factors. Eur J Cancer 1998; 34 (12): 1857–64
Cosler LE, Calhoun EA, Agboola O, et al. Effects of indirect and additional direct costs on the risk threshold for prophylaxis with colony-stimulating factors in patients at risk for severe neutropenia from cancer chemotherapy. Pharmacotherapy 2004; 24 (4): 488–94
Doorduijn JK, Buijt I, van der Holt B, et al. Economic evaluation of prophylactic granulocyte colony stimulating factor during chemotherapy in elderly patients with aggressive non-Hodgkin’s lymphoma. Haematologica 2004; 89(9): 1109–17
Bojke L, Sculpher M, Stephens R, et al. Cost effectiveness of increasing the dose intensity of chemotherapy with granulocyte colony-stimulating factor in small-cell lung cancer: based on data from the Medical Research Council LU19 trial. Pharmacoeconomics 2006; 24 (5): 443–52
Timmer-Bonte JN, Adang EM, Smit HJ, et al. Cost-effectiveness of adding granulocyte colony-stimulating factor to primary prophylaxis with antibiotics in small-cell lung cancer. J Clin Oncol 2006; 24 (19): 2991–7
Eldar-Lissai A, Cosler LE, Culakova E, et al. Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health 2008; 11 (2): 172–9
Timmer-Bonte JN, Adang EM, Termeer E, et al. Modeling the cost effectiveness of secondary febrile neutropenia prophylaxis during standard-dose chemotherapy. J Clin Oncol 2008; 26 (2): 290–6
Ramsey SD, Liu Z, Boer R, et al. Cost-effectiveness of primary versus secondary prophylaxis with pegfilgrastim in women with early-stage breast cancer receiving chemotherapy. Value Health 2009; 12 (2): 217–25
Danova M, Chiroli S, Rosti G, et al. Cost-effectiveness of pegfilgrastim versus six days of filgrastim for preventing febrile neutropenia in breast cancer patients. Tumori 2009; 95 (2): 219–26
Heaney ML, Toy EL, Vekeman F, et al. Comparison of hospitalization risk and associated costs among patients receiving sargramostim, filgrastim, and pegfilgrastim for chemotherapy-induced neutropenia. Cancer 2009; 115 (20): 4839–48
Liu Z, Doan QV, Malin J, et al. The economic value of primary prophylaxis using pegfilgrastim compared with filgrastim in patients with breast cancer in the UK. Appl Health Econ Health Policy 2009; 7 (3): 193–205
Lyman GH, Lalla A, Barron RL, et al. Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin Ther 2009; 31 (5): 1092–104
Raftery J. NICE: faster access to modern treatments? Analysis of guidance on health technologies. BMJ 2001; 323 (7324): 1300–3
Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw 2008; 6(2): 109–18
Lyman GH, Delgado DJ. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 2003; 98 (11): 2402–9
Haithcox S, Ramnes CR, Lee H, et al. The impact of frequent injections for hematopoietic growth factor support on patients receiving chemotherapy: an observational study. BMC Nurs 2003; 2 (1): 2
Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol 2003; 21 (24): 4524–31
Price N, Jain VK, Lyman GH. Prophylactic pegfilgrastim significantly reduces febrile neutropenia during moderately myelosuppressive chemotherapy. Support Cancer Ther 2004; 1 (4): 207–9
Clark OA, Lyman GH, Castro AA, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol 2005; 23 (18): 4198–214
Song X, Fowler R, Ruiz K, et al. Impact of neutropenic complications on short-term disability in patients with cancer receiving chemotherapy. J Med Econ 2009; 12 (2): 154–63
Fortner BV, Tauer K, Zhu L, et al. Medical visits for chemotherapy and chemotherapy-induced neutropenia: a survey of the impact on patient time and activities. BMC Cancer 2004; 4: 22
Jelkmann W. Biosimilar epoetins and other ‘follow-on’ biologics: update on the European experiences. Am J Hematol 2010; 85 (10): 771–80
Skrlin A, Radic I, Vuletic M, et al. Comparison of the physicochemical properties of a biosimilar filgrastim with those of reference filgrastim. Biologicals 2010; 38 (5): 557–66
Waller CF, Bronchud M, Mair S, et al. Pharmacokinetic profiles of a biosimilar filgrastim and Amgen filgrastim: results from a randomized, phase I trial. Ann Hematol 2010; 89 (9): 927–33
Mellstedt H. Implications of the development of biosimilars for cancer treatment. Future Oncol 2010; 6 (7): 1065–7
Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol 2008; 19 (3): 411–9
Lyman GH, Kuderer NM, Crawford J, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 2011; 117 (9): 1917–27
Acknowledgements
No funding was provided for the preparation of this article.
Dr Lyman is principal investigator on a research grant to Duke University from Amgen. Dr Hirsch declares no conflicts of interest. Dr Lyman conceived the idea for the paper. Drs Hirsch and Lyman designed and provided the analysis, and wrote the paper. Both authors approved the accuracy of the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirsch, B.R., Lyman, G.H. Pharmacoeconomics of the Myeloid Growth Factors. PharmacoEconomics 30, 497–511 (2012). https://doi.org/10.2165/11590130-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11590130-000000000-00000