Abstract
Background and objective: Enterally administered low-dose ketamine is being used increasingly to treat pain states. However, suitable oral or sublingual formulations are not available. The objective of the study was to develop a lozenge formulation of ketamine for use in patients with neuropathic pain, and to investigate its storage stability and bioavailability after oral or sublingual administration.
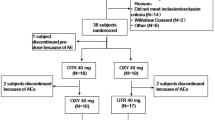
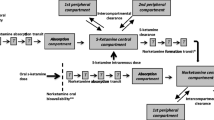
Methods: A lozenge containing 25mg of ketamine was formulated and manufactured in a hospital pharmacy setting. Stability was assessed by high-performance liquid chromatography (HPLC) during storage at 25°C or 2–8°C for up to 14 weeks. Bioavailability after both oral and sublingual administration was evaluated in six patients with chronic neuropathic pain. Ketamine and its metabolite norketamine in plasma were measured by HPLC.
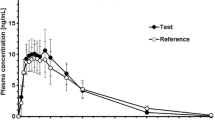
Results: The lozenge formulation was chemically stable for at least 14 weeks. Oral and sublingual bioavailabilities [median (interquartile range)] were 24% (17–27%) and 24% (19–49%), respectively. There was substantial metabolism to norketamine for both routes. The mean norketamine/ketamine area under the plasma concentration-time curve from baseline to 8 hours ratios were 5 and 2.1 after oral or sublingual administration, respectively.
Conclusion: The ketamine lozenge showed acceptable storage stability. Bioavailability was sufficiently high and reproducible to support its use in routine pain management. There was extensive first-pass conversion to norketamine. Efficacy studies are warranted to evaluate sublingual and oral administration of our new lozenge formulation of ketamine in patients with chronic pain states. Investigation of the role of the metabolite norketamine, which is also an NMDA receptor antagonist, is particularly important because this may contribute significantly to clinical efficacy.



Similar content being viewed by others
References
Reich DL, Silvay G. Ketamine: an update on the 1st 25 years of clinical experience. Can J Anaesth 1989; 36: 186–97
Walker SM, Cousins MJ. Reduction in hyperalgesia and intrathecal morphine requirements by low-dose ketamine infusion. J Pain Symptom Manage 1997; 14: 129–33
Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg 2003; 97: 1730–9
Sang CN. NMDA-receptor antagonists in neuropathic pain: experimental methods to clinical trials. J Pain Symptom Manage 2000; 19: S21–5
Price DD, Mayer DJ, Mao J, et al. NMDA-receptor antagonists and opioid receptor interactions as related to analgesia and tolerance. J Pain Symptom Manage 2000; 19: S7–11
Sator-Katzenschlager S, Deusch E, Maier P, et al. The long-term antinociceptive effect of intrathecal S(+)-ketamine in a patient with established morphine tolerance. Anesth Analg 2001; 93: 1032–4
Eilers H, Philip LA, Bickler PE, et al. The reversal of fentanyl-induced tolerance by administration of “small-dose” ketamine. Anesth Analg 2001; 93: 213–4
Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg 2004; 99: 482–95
Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci 1982; 71: 539–42
Hijazi Y, Boulieu R. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 2002; 30: 853–8
Kannan TR, Saxena A, Bhatnagar S, et al. Oral ketamine as an adjuvant to oral morphine for neuropathic pain in cancer patients. J Pain Symptom Manage 2002; 23: 60–5
Fitzgibbon EJ, Hall P, Schroder C, et al. Low dose ketamine as an analgesic adjuvant in difficult pain syndromes: a strategy for conversion from parenteral to oral ketamine. J Pain Symptom Manage 2002; 23: 165–70
Enarson MC, Hays H, Woodroffe MA. Clinical experience with oral ketamine. J Pain Symptom Manage 1999; 17: 384–6
Gross AS, Nicolay A, Eschalier A. Simultaneous analysis of ketamine and bupivacaine in plasma by high-performance liquid chromatography. J Chromatog B 1999; 728: 107–15
Hijazi Y, Bolon M, Boulieu R. Stability of ketamine and its metabolites norketamine and dehydronorketamine in human biological samples. Clin Chem 2001; 47: 1713–5
Thomann P. Non-compartmental analysis methods manual. In: Heinzel G, Woloszcak R, Thomann P, editors. TopFit 2.0 pharmacokinetic and pharmacodynamic data analysis system for the PC. Stuttgart: Gustav Fischer, 1993: 5–66
Heinzel G, Tanswell P. Compartmental analysis methods manual. In: Heinzel G, Woloszcak R, Thomann P, editors. Topfit 2.0 pharmacokinetic and pharmacodynamic data analysis system for the PC. Stuttgart: Gustav Fischer, 1993: 5–140
Yanagihara Y, Ohtani M, Kariya S, et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos 2003; 24: 37–43
Hijazi Y, Bodonian C, Salord F, et al. Pharmacokinetic-pharmacodynamic modelling of ketamine in six neuro-traumatised intensive care patients. Clin Drug Invest 2003; 23: 605–9
Yanagihara Y, Ohtani M, Matsumoto M, et al. Preparation of ketamine tablets for treatment of patients with neuropathic pain. Yakugaku Zasshi 1999; 119: 980–7
Grant IS, Nimmo WS, Clements JA. Pharmacokinetics and analgesic effects of i.m. and oral ketamine. Br J Anaesth 1981; 53: 805–10
Ebert B, Mikkelsen S, Thorkildsen C, et al. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol 1997; 333: 99–104
Shimoyama M, Shimoyama N, Gorman AL, et al. Oral ketamine is antinociceptive in the rat formalin test: role of the metabolite, norketamine. Pain 1999; 81: 85–93
Edwards SR, Mather LE. Tissue uptake of ketamine and norketamine enantiomers in the rat: indirect evidence for extrahepatic metabolic inversion. Life Sci 2001; 69: 2051–66
Bushnell TG, Craig J. Response of chronic neuropathic pain syndromes to ketamine: a role for norketamine [letter]? Pain 1995; 60: 115
Acknowledgements
The study was funded by a project grant from the Australian and New Zealand College of Anaesthetists (ANZCA), Melbourne, Australia. The grant had no influence on the way the study was conducted.
The role of the Pharmacy of Royal Perth Hospital, Perth, Australia in the development of the lozenge and the editorial assistance of Falk Reinholz, University of Western Australia, Perth, Australia in the preparation and finalization of this manuscript are kindly acknowledged.
The authors have no conflicts of interest that are directly related to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chong, C., Schug, S.A., Page-Sharp, M. et al. Development of a Sublingual/Oral Formulation of Ketamine for Use in Neuropathic Pain. Clin. Drug Investig. 29, 317–324 (2009). https://doi.org/10.2165/00044011-200929050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200929050-00004