Abstract
Man and higher primates have adrenals that secrete large amounts of dehydroepiandrosterone (DHEA) [prasterone] and its sulphate (DHEAS) [PB 008]. A remarkable feature of plasma DHEAS levels in humans is their great decrease with aging. Researchers have postulated that this age-related decline of DHEAS levels may explain some of the degenerative changes associated with aging. Moreover, administration of DHEA to laboratory animals has demonstrable beneficial effects such as prevention of diabetes mellitus, obesity, cancer, heart disease and positive immunomodulator effects. However, in rodents DHEA(S) circulating levels are so low that it is impossible to detect any significant age-related decrease. Therefore results from rodent experiments are not relevant to human beings.
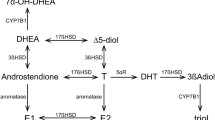
Three mechanisms of action of DHEA(S) have been identified. DHEA and DHEAS are precursors of testosterone and estradiol, DHEAS is a neurosteroid which modulates neuronal excitability via specific interactions with neurotransmitter receptors and DHEA is an activator of calcium-gated potassium channels.
Randomised, placebo-controlled clinical trials which included healthy individuals aged 60 years and over treated with (near) physiological doses of DHEA (50–100 mg/day) have yielded very few positive results. Impact of DHEA replacement treatment was assessed on mood, well being, cognitive and sexual functions, bone mass, body composition, vascular risk factors, immune functions and skin. The major limitations of these trials were their short duration (maximum 1 year) and the low number of study participants involved (maximum 280).
Many elderly people in western countries take DHEA without medical supervision. In the US, DHEA is even classified as food supplement. At present there is no scientific evidence to recommend DHEA replacement in the elderly. Further studies are needed to form conclusions about the efficacy and the safety of DHEA replacement in elderly, and to better understand the mechanisms of action of DHEA at the molecular and cellular levels.





Similar content being viewed by others
References
Nawata H, Yanase T, Goto K, et al. Mechanisms of action of anti-aging DHEA-S and the replacement of DHEA-S. Mech Ageing Devel 2002; 123: 1101–6
Svec F, Porter JR. The actions of exogenous dehydroepiandrosterone in experimental animals and humans. Proc Exp Biol Med 1998; 218(3): 174–91
Tomera JF. Dehydroepiandrosteron and aging. Drugs Today 1996; 32: 453–61
Baulieu EE, Corpechot C, Dray F, et al. Adrenal-secreted “androgen:” dehydroisoandrosterone sulfate. Its metabolism and a tentative generalisation on the metabolism of other steroid conjugates in man. Recent Prog Horm Res 1965; 21: 411–500
Robert KD, Vande Wiele RL, Lieberman. The conversion in vivo of dehydroisoandrosterone sulfate to androsterone and etiocholanolone glucuronidates. J Biol Chem 1961; 236: 2213–5
Rosenfeld RS, Hellman L, Gallagher TF. Metabolism and interconversion of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J Clin Endocrinol Metab 1972; 35(2): 187–93
Bird CE, Murphy J, Borooman K, et al. Dehydroepiandrosterone: kinetics of metabolism in normal men and women. J Clin Endocrinol Metab 1978; 47: 818–22
Adams JB. Control of secretion and the function of C19-delta 5-steroids of the human adrenal gland. Mol Cel Endocrinol 1985; 41(1): 1–17
Poortman J, Prenen JA, Schwarz, et al. Interaction of delta-5-androstene-3beta, 17beta-diol with estradiol and dihydrostestosterone receptors in human myometrial and mammary cancer tissue. J Clin Endocrinol Metab 1975; 40(3): 373–9
Labrie F, Luu-The V, Lin SX, et al. Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol 2000; 25(1): 1–16
Horton R, Tait JF. In vivo conversion of dehydroisoandrosterone to plasma androstenedione and testosterone in man. J Clin Endocrinol Metab 1967; 27(1): 79–88
Belisle S, Schiff I, Tulchinsky D. The use of constant infusion of unlabeled dehydroepiandrosterone for the assessment of its metabolic clearance rate, its half-live, and its conversion into estrogens. J Clin Endocrinol Metab 1980; 50: 117–21
Bird CE, Masters V, Clarks AF. Dehydroepiandrosterone sulfate: kinetics of metabolism in normal young men and women. Clin Invest Med 1984; 7: 119–22
Haning RV, Flood CA, Hackett RJ, et al. Metabolic clearance rate of dehydroepiandrosterone sulfate, its metabolism to testosterone, and its intra follicular metabolism to dehydroepiandrosterone, androstenedione, testosterone and dihydrotestosterone in vivo. J Clin Endocrinol Metab 1991; 72(5): 1088–95
Baulieu EE. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocrinol Metab 1996; 81(9): 3147–51
Parker LN, Odell WD. Control of adrenal androgen secretion. Endocrine Rev 1980; 1(4): 392–410
Hornsby PJ. Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Ann N Y Acad Sci 1995; 774: 29–46
Nestler JE. Advances in understanding the regulation and biologic actions of dehydroepiandrosterone. Curr Opinion Endocrinol Diabet 1996; 3: 202–11
Schlienger JL, Perrin AE, Goichot B. DHEA: célèbre et méconnue. Rev Méd Interne 2002; 23: 436–46
Allolio B, Art W. DHEA treatment: myth or reality? Trends Endocrinol Metab 2002; 13: 288–94
Johnson MD, Bebb RA, Sirrs SM. Uses of DHEA in aging and other disease states. Ageing Res Rev 2002; 1: 29–41
Moghissi E, Ablan F, Horton R. Origin of plama androstanediol glucuronide in men. J Clin Endocrinol Metab 1984; 59(3): 417–21
Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab 1976; 42(2): 247–53
Labrie F. Intracrinology. Mol Cell Endocrinol 1991; 78: C113–8
Belanger B, Belanger A, Labrie F, et al. Comparison of residual C-19 steroids in plasma and prostatic tissue of human, rat and guinea pig after castration: unique importance of extratesticular androgens in men. J Steroid Biochem 1989; 32(5): 695–8
Robel P, Baulieu EE. Dehydroepiandrosterone (DHEA) is a neuroactive neurosteroid. Ann N Y Acad Sci 1995; 774: 82–109
Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci 1999; 22(9): 410–6
Vallee M, Mayo W, Le Moal M. Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Res Rev 2001; 37: 301–12
Monnet FP, Mahe V, Robel P, et al. Neurosteroids, via σ receptors, modulate the (3H)norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci U S A 1995; 92(9): 3774–8
Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Rev 1999; 30: 264–88
Farrukh IS, Peng W, Orlinska U, et al. Effects of dehydroepiandrosterone on hypoxic pulmonary vasoconstriction: a Ca2+ activated K+-channel opener. Am J Physiol 1998; 274: L186–95
Peng W, Hoidal JR, Farrukh IS. Role of a novel KCa opener in regulating K+ channels of hypoxic human pulmonary vascular cells. Am J Respir Cell Mol Biol 1999; 20: 737–45
Hampl V, Bibova J, Povysilova V, et al. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J 2003; 21: 862–5
Bonnet S, Dumas-de-la-Roque E, Begueret H, et al. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci U S A 2003; 100(16): 9488–93
Okabe T, Haji M, Takanagi R, et al. Up-regulation of high-affinity dehydroepiandrosterone binding activity by dehydroepiandrosterone in activated human T lymphocytes. J Clin Endocrinol Metab 1995; 80(10): 2993–6
Meikle AW, Dorchuck RW, Araneo BA, et al. The presence of a dehydroepiandrosterone-specific receptor binding complex in murine T cells. J Steroid Biochem Mol Biol 1992; 42(3/4): 293–304
Birkenhäger-Gillesse EG, Derksen J, Lagaay AM. Dehydroepiandrosterone sulphate (DHEAS) in the oldest old aged 85 and over. Ann N Y Acad Sci 1996; 774: 543–52
Laughlin GA, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging an adrenal hormone levels: the Rancho Bernardo Study. J Clin Endocrinol Metab 2000; 85(10): 3561–8
Orentreich N, Brind JL, Rizer RL, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 1984; 59(3): 551–5
Ravaglia G, Forti P, Maioli M, et al. The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest-old: results from an Italian study on healthy free-living over-ninety-year-olds. J Clin Endocrinol Metab 1996; 81(3): 1173–8
Mazat L, Lafont S, Berr C, et al. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc Natl Acad Sci U S A 2001; 98(14): 8145–50
Orentreich N, Brind JL, Vogelman JH, et al. Long term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab 1992; 75(4): 1002–4
Legrain S, Massien C, Lahlou N, et al. Dehydroepiandrosterone replacement administration: pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab 2000; 85(9): 3208–17
Rozenberg S, Bosson D, Peretz A, et al. Serum levels of gonadotrophins and steroid hormones in the post-menopause and later life. Maturitas 1988; 10: 215–24
Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma Cortisol. J Clin Endocrinol Metab 1996; 81(7): 2468–73
Lupien SJ, Nair NP, Briere S, et al. Increased Cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev Neurosci 1999; 10(2): 117–39
Parker CR. Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 1999; 64: 640–7
Liu CH, Laughlin GA, Fischer UG, et al. Marked attenuation of ultradian and circadian rhythms of dehydroepiandrosterone in post menopausal women: evidence for reduced 17,20 desmolase activity. J Clin Endocrinol Metab 1990; 71(4): 900–6
Ohashi M, Kato K, Nawata H, et al. Adrenocortical responsiveness to graded ACTH infusions in normal young and elderly human subjects. Gerontology 1986; 32: 43–51
Vermeulen A, Deslypere JP, Schelfhout W, et al. Adrenocortical function in old age: response to acute adrenocorticotropin stimulation. J Clin Endocrinol Metab 1982; 54: 187–95
Deuschle M, Gotthardt U, Schweiger U, et al. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases its diurnal and its amplitude flattens. Life Sci 1997; 61(22): 2239–46
Parker CR, Slayden SM, Azziz RM, et al. Effects of aging an adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J Clin Endocrinol Metab 2000; 85(1): 48–54
Parker CR, Mison RL, Brissie RM, et al. Aging alters zonation in the adrenal cortex of men. J Clin Endocrinol Metab 1997; 82(11): 3898–901
Carlström K, Brody S, Lunell NO, et al. Dehydroepiandrosterone sulphate and dehydroepiandrosterone in serum: differences related to age and sex. Maturitas 1988; 10: 294–306
De Peretti E, Forest MG. Patterns of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: evidence for testicular production. J Clin Endocrinol Metab 1978; 47: 572–7
Zumoff B, Bradlow HL. Sex difference in the metabolism of dehydroepiandrosterone sulfate. J Clin Endocrinol Metab 1980; 51(2): 334–6
Legrain S, Berr C, Frenoy N, et al. Dehydroepiandrosterone sulfate in a long-term care aged population. Gerontology 1995; 41: 343–51
Thomas G, Frenoy N, Legrain S, et al. Serum dehydroepiandrosterone sulfate levels as an individual marker. J Clin Endocrinol Metab 1994; 79(5): 1273–6
Meikle AW, Stringham MG, Woodward MG, et al. Heritability of variation of plasma cortisol levels. Metabolism 1988; 37: 514–7
Rotter JI, Wong FL, Lifrak ET, et al. A genetic component to the variation of dehydroepiandrosterone sulfate. Metabolism 1985; 34: 731–6
Parker LN, Eugene J, Farber D, et al. Dissociation of adrenal androgen and cortisol levels in acute stress. Horm Metab Res 1985; 17: 209–12
Parker LN, Levine ER, Lefrak ET. Evidence of adrenocortical adaptatation to severe illness. J Clin Endocrinol Metab 1985; 60(5): 947–55
Semple CG, Gray CE, Beastall GH. Adrenal androgens and illness. Acta Endocrinol (Copenh) 1987; 116: 155–60
Wade CE, Lindberg JS, Cockrell JL, et al. Upon-admission adrenal steroidogenesis is adapted to the degree of illness in intensive care unit patients. J Clin Endocrinol Metab 1988; 67(2): 223–7
Deighton C, Watson MJ, Walker DJ. Sex hormones in postmenopausal HLA-Identical rhumatoid arthritis discordant sibling pairs. J Rheumatol 1992; 19: 1663–7
Rudman D, Shetty KR, Mattson DE. Plasma dehydroepiandrosterone sulfate in nursing home men. J Am Geriatr Soc 1990; 38(4): 421–7
Berkman LF, Seeman TE, Albert M, et al. High, usual, and impaired functioning in community-dwelling older men and women: findings of the Macarthur Foundation Research network on successful aging. J Clin Epidemiol 1993; 46(10): 1129–40
Barrett-Connor E, Khaw KT, Yen SSC. A prospective study of dehydroepiandrosterone sulfate, mortality and cardiovascular disease. N Engl J Med 1986; 315(24): 1519–24
Barrett-Connor E, Khaw KT. Absence of an inverse relation of DHEAS with cardiovascular mortality in postmenopausal women [letter]. N Engl J Med 1987; 317: 711
Arlt W, Hass J, Callies F, et al. Biotransformation of oral dehydroepiandrosterone in elderly men: significant increase in circulating estrogens. J Clin Endocrinol Metab 1999; 84: 2170–6
Oelkers W. Dehydroepiandrosterone for adrenal insufficiency. N Engl J Med 1999; 341(14): 1073–4
Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335(16): 1206–12
Tomlinson JW, Holden N, Hills RK Association between premature mortality and hypopuitarism. Lancet 2001; 357: 425–31
Arlt W, Callies F, van Vlijmen JC, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med 1999; 341(14): 1013–20
Hunt PJ, Gurnell EM, Huppert FA, et al. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. J Clin Endocrinol Metab 2000; 85(12): 4650–6
Johannsson G, Burman P, Wirén L, et al. Low dose dehydroepiandrosterone affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab 2002; 87(5): 2046–52
Majewska MD. Neuronal actions of dehydroepiandrosterone: possible roles in brain development aging, memory, and affect. Ann N Y Acad Sci 1995; 774: 111–20
Morales AJ, Nolan JJ, Nelson JC, et al. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab 1994; 78(6): 1360–7
Arlt W, Callies F, Koehler I, et al. Dehydroepiandrosterone supplementation in healthy men with an age-related decline of dehydroepiandrosterone secretion. J Clin Endocrinol Metab 2001; 86(10): 4686–92
Flynn MA, Weaver-Osterholtz D, Sharpe-Timms KL, et al. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab 1999; 84(5): 1527–33
+kerk J, Huppert FA, Herbert J. Salivary Cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology 2001; 26: 591–612
Wolf OT, Neuman O, Hellhammer DH, et al. Effects of a two-week physiological dehydroepiandrosterone substitution on cognitive performance and well-being in healthy elderly women and men. J Clin Endocrinol Metab 1997; 82: 2363–7
Wolf OT, Naumann D, Hellhammer DH, et al. Effects of dehydroepiandrosterone replacement in elderly men on eventrelated potentials, memory, and well-being. J Gerontol A Biol Sci Med Sci 1998; 53A(5): M385–90
Dean CE. Prasterone (DHEA) and mania. Ann Pharmacother 2000; 34(12): 1419–22
Kline MD. Mania onset while using dehydroepiandrosterone [letter]. Am J Psychiatry 1999; 156(6): 971
Reiter WJ, Pycha A, Schatzl G, et al. Dehydroepiandrosterone in the treatment of erectile dysfunction: a prospective, double-blind, randomized, placebo-controlled study. Urology 1999; 53(3): 590–5
Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of DHE-Age study to a sociobiomedical issue. Proc Natl Acad Sci U S A 2000; 97(8): 4279–84
Barnarht KT, Freeman E, Grisso JA, et al. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab 1999; 84(11): 3896–902
Bloch M, Schmidt PJ, Danacau MA, et al. Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry 1999; 45: 1533–41
Wolkowitz OM, Kramer JH, Reus VI, et al. DHEA treatment of Alzheimer’s disease: a randomized, double-blind, placebo-controlled study. Neurology 2003; 60(7): 1071–6
Ben-David M, Dikstein S, Bismuth G, et al. Anti-cholesterolemic effect of dehydroepiandrosterone in rats. Proc Soc Exp Biol Med 1967; 125: 1136–40
Kurzman ID, Macewen EG, Haffa ALM. Reduction in body weight and cholesterol in spontaneously obese dogs by dehydroepiandrosterone. Int J Obes 1990; 14(2): 95–104
Arad Y, Badimon JO, Badimon L, et al. Dehydroepiandrosterone feedings prevents aortic fatty streak formation and cholesterol accumulation in cholesterol-fed rabbit. Arteriosclerosis 1989; 9: 159–66
Gordon GB, Bush DE, Weisman HF. Reduction of atherosclerosis by administration of dehydroepiandrosterone: a study in the hypercholesterolemic New Zealand white rabbit with aortic intimal injury. J Clin Invest 1988; 82: 712–20
MacEwen EG, Kurzman ID. Obesity in the dog: role of the adrenal steroid dehydroepiandrosterone (DHEA). J Nutr 1991; 121: S51–5
Yen TT, Allan JA, Pearson DV, et al. Prevention of obesity in Avy/a mice by dehydroepiandrosterone. Lipids 1977; 12(5): 409–13
Berr C, Lafont S, Debuire B, et al. Relationship of dehydroepiandrosterone sulfate (DHEAS) in the elderly with functional, psychological and mental status, and short-term mortality: a French community-based study. Proc Natl Acad Sci U S A 1996; 93(23): 13410–5
LaCroix AZ, Yano K, Reed DM. Dehydroepiandrosterone sulfate, incidence of myocardial infarction, and extent of atherosclerosis in men. Circulation 1992; 86(5): 1529–35
Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab 2001; 86(9): 4171–7
Barrett-Connor E, Goodman-Gruen D. Dehydroepiandrosterone sulfate does not predict cardiovascular death in post menopausal women. The Rancho Bernardo Study. Circulation 1995; 91(6): 1757–60
Hautanen A, Manttari M, Manninen V, et al. Adrenal androgens and testosterone as coronary risk factors in the Helsinki Heart Study. Atherosclerosis 1994; 105(2): 191–200
Herrington DM, Gordon GB, Achuff SC, et al. Plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate in patients undergoing diagnostic coronary angiography. J Am Coll Cardiol 1990; 16(6): 862–70
Herrington DM, Nanjee N, Achuff SC, et al. Dehydroepiandrosterone and cardiac allograft vasculopathy. J Heart Lung Transplant 1996; 15 (1 Pt 1): 88–93
Barrett-Connor E, Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio and non insulin-dependent diabetes in postmenopausal women: the Rancho Bernardo study. J Clin Endocrinol Metab 1996; 81(1): 59–64
Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol 2000; 53(5): 561–8
Yen SSC, Morales AJ, Khorram O. Replacement of dhea in aging men and women: potential remedial effects. Ann N Y Acad Sci 1995; 774: 128–42
Morales AJ, Haubrich RH, Hwang JY, et al. The effect of six months treatment with a 100mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol 1998; 49(4): 421–32
Diamond P, Cusan L, Gomez JL, et al. Metabolic effect of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. Endocrinology 1996; 150: S43–50
Casson PR, Faquin LC, Stentz FB, et al. Replacement of dehydroepiandrosterone enhances T-lymphocyte insulin binding in postmenopausal women. Fertil Steril 1995; 63(5): 1027–31
Labrie F, Diamond P, Cusan L, et al. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina and endometrium in postmenopausal women. J Clin Endocrinol Metab 1997; 82(10): 3498–505
Khan AJ, Halloran B. Dehydroepiandrosterone supplementation and bone turnover in middle-aged to elderly men. J Clin Endocrinol Metab 2002; 87(4): 1544–9
Percheron G, Hogrel JY, Denot-Ledunois S, et al. Effects of one-year oral administration of DHEA to 60–80 years old individuals on muscle function and cross sectional area: a double-blind placebo controlled. Arch Intern Med 2003; 163(6): 720–7
Franceschi C, Monti D, Sansoni P, et al. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today 1995; 16(1): 12–6
Murasko DM, Weiner P, Kaye D. Decline in mitogen induced proliferation of lymphocytes with increasing age. Clin Exp Immunol 1987; 70: 440–8
Paganelli R, Quinti I, Fagiolo U, et al. Changes in circulating B cells and immunoglobulin classes and subclasses in a healthy aged population. Clin Exp Immunol 1992; 90: 351–4
Sansoni P, Cossarizza A, Brianti V, et al. Lymphocytes subsets and natural killer cell activity in healthy old people and centenarians. Blood 1993; 82(9): 2767–73
Spencer NFL, Poynter ME, Hennebold JD, et al. Does DHEAS restore immune competence in aged animals through its capacity to function as a natural modulator of peroxisome activities? Ann N Y Acad Sci 1995; 774: 200–16
Daynes RA, Araneo BA, Ershler WB, et al. Altered regulation of IL6 production with normal aging: probable linkage to the age-associated decline in dehydroepiandrosterone and its sulfate derivative. Immunology 1993; 150: S219–30
Ershler WB, Sun WH, Binkley N, et al. Interleukine 6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res 1993; 12: 225–30
Krishnaraj R, Blandford G. Age-associated alterations in human Natural Killer cells. Clin Immunol Immunopathol 1987; 45: 268–85
Murasko DM, Nelson BJ, Silver R, et al. Immunologic response in an elderly population with a mean age of 85. Am J Med 1986; 81: 612–8
Carson PJ, Nichol KL, O’Brien J, et al. Immune function and vaccine responses in healthy advanced elderly patients. Arch Intern Med 2000; 160(13): 2017–24
Franceschi C, Valensin S, Fagnoni F, et al. Biomarkers of immunosenescence within an evolutionary perspective: the challenge of heterogeneity and the role of antigenic load. Exp Gerontol 1999; 34: 911–21
Daynes RA, Araneo BA, Hennebold J, et al. Steroids as regulators of the mammalian immune response. J Invest Dermatol 1995; 105: 14S–9S
Araneo BA, Woods ML, Daynes RA. Reversal of the immunosenescent phenotype by dehydroepiandrosterone: hormone treatment provides an adjuvant effect on the immunization of aged mice with recombinant hepatitis B surface antigen. J Infect Dis 1993; 167: 830–40
Loria RM, Inge TH, Cook SS, et al. Protection against acute lethal viral infection with the native dehydroepiandrosterone (DHEA). J Med Virol 1988; 26: 301–14
Loria RM, Regelson W, Padgett DA. Immune response facilitation to virus and bacterial infections with dehydroepiandrosterone (DHEA). In: Kalimi M, Regelson W, editors. The biologic role of Dehydroepiandrosterone (DHEA). New York: Walter de Gruyter & Co, 1990: 101–26
Chang DM, Lan JL, Lin HY, et al. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002; 46(11): 2924–7
Petri MA, Lahita RG, Van Vollenhoven RF, et al. Effect on prasterone on corticosteroid requirements of women with systemic lupus erythematosus: a double, randomized, placebo-controlled trial. Arthritis Rheum 2002; 46(7): 1820–9
Van Vollenhoven RF, Engelman EG, Mc Guire JL. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum 1994; 37(9): 1305–10
Van Vollenhoven RF, Engleman EG, Mc Guire JL. Dehydroepiandrosterone in systemic lupus erythematosus: results of a double-blind, placebo-controlled, randomized clinical trial. Arthritis Rheum 1995; 38: 1826–31
Van Vollenhoven RF, Park JL, Genovese MC, et al. A double-blind, placebo-controlled, clinical trial of dehydroepiandrosterone in severe systemic lupus erythematosus. Lupus 1999; 8(3): 181–7
Casson PR, Andersen NA, Herrod HG, et al. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. Am J Obstet Gynecol 1993; 169: 1536–9
Evans TG, Judd ME, Dowell T, et al. The use of dehydroepiandrosterone sulfate as an adjuvant in tetanus and influenza vaccination of the elderly. Vaccine 1996; 14(16): 1531–7
Danenberg HD, Ben-Yehuda A, Zakay-Rones Z, et al. Dehydroepiandrosterone treatment is not beneficial to the immune response to influenza in elderly subjects. J Clin Endocrinol Metab 1997; 82(9): 2911–4
Degelau J, Guay D, Hallgren H. The effect of DHEAS on influenza vaccination in aging adults. J Am Geriatr Soc 1997; 45(6): 747–51
Shah MG, Maibach HI. Estrogen and skin: an overview. Am J Clin Dermatol 2001; 2(3): 143–50
Ebeling P, Koivisto VA. Physiological importance of dehydroepiandrosterone. Lancet 1994; 343: 1479–81
Stoll BA. Dietary supplements of dehydroepiandrosterone in relation to breast cancer risk. Eur J Clin Nutr 1999; 53(10): 771–5
Maggiolini M, Donze O, Jeannin E, et al. Adrenal androgens stimulate proliferation of breast cancer cells as direct activators of estrogen receptor α1. Cancer Res 1999; 59: 4864–9
Morris KT, Toth Fejel S, Schmidt J, et al. High dehydroepiandrosterone-sulfate predicts breast cancer progression during new aromatase inhibitor therapy and stimulates breast cancer cell growth in tissue culture: a renewed role for adrenalectomy. Surgery 2001; 130(6): 947–53
Schmitt M, Klinga K, Schnarr B, et al. Dehydroepiandrosterone stimulates prolidferation and gene expression in MCF-7 cells after conversion to estradiol. Mol Cell Endocrinol 2001; 173: 1–13
Tan J, Sharief Y, Hamil G, et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol 1997; 11: 450–9
Herbert J. The age of dehydroepiandrosterone. Lancet 1995; 345(8959): 1193–4
Weksler ME. Hormone replacement for men. BMJ 1996; 312(7035): 859–60
Acknowledgements
Sylvie Legrain and Laurence Girard thank Chantal and Michel Marait for assistance in preparation of this manuscript.
The authors have no conflicts of interest directly relevant to the content of this paper and have provided no information on sources of funding directly relevant to the content of this review.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Legrain, S., Girard, L. Pharmacology And Therapeutic Effects of Dehydroepiandrosterone In Older Subjects. Drugs Aging 20, 949–967 (2003). https://doi.org/10.2165/00002512-200320130-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200320130-00001