Continuous intraoperative vagus nerve stimulation for monitoring of recurrent laryngeal nerve during minimally invasive esophagectomy
Introduction
Esophagectomy together with extended mediastinal lymphadenectomy has been shown to be beneficial for patients with esophageal cancer, in particular squamous cell cancer, which is the predominant cell type in Asia. Because of the propensity of nodal spread along the recurrent laryngeal nerves (RLN), bilateral RLN nodal dissection is an important component of the operation. The RLN’s are at risk of injury and the incidence of vocal cord paralysis after esophagectomy can be as high as 60% to 70% (1,2). In thyroidectomy, monitoring of RLN may reduce the chance of nerve injury. Conventional nerve monitoring requires identification and active intermittent stimulation of the RLN. The nerve can be injured in between stimulations. A new technique of continuous intraoperative nerve monitoring (CIONM) works through automatic periodic stimulation (APS) of the vagus nerve. The vagus nerve is first isolated, and then an electrode is wrapped around it. Periodic electrical stimulation is applied to the RLN. Completeness of the circuit is ascertained when the electrical signal is picked by an electrode on the endotracheal tune. It detects the amplitude of the electromyography (EMG) of the vocalis muscle as well as its latency of nerve conduction. Reduction in amplitude and increase in latency of the laryngeal EMG signifies imminent injury to the RLN. In thyroidectomy, this system has been shown to be useful (3,4). We describe the adaptation of a commercially available continuous nerve monitoring equipment—NIM 3.0 with APS (Medtronic Inc., Jacksonville, FL, USA) for use in video-assisted thoracoscopic (VATS) minimally invasive esophagectomy (MIE). A pilot study of ten patients has been published recently (5).
Patient selection and workup
Extended lymphadenectomy is associated with increased morbidity; many surgeons would be selective in its application only in good risk patients. After neoadjuvant chemoradiotherapy, many would also shy away from RLN dissection, especially in the context of VATS esophagectomy. Radiotherapy may result in more fibrosis with obscured tissue planes and it is hard to differentiate cancer infiltration from post radiation fibrosis. In other cases, the tissue may actually become more edematous, easing dissection (6). In the TIME (Traditional Invasive vs. MIE) trial, the primary end-point is difference in pulmonary infection rates between open and MIE. The pulmonary complication rate and the RLN palsy rate were lower in the minimally invasive group while other complications and mortality rates were not significantly different. Bilateral RLN nodal dissection however was not routinely performed (7). Our patients were unselected; the intent was to perform bilateral RLN dissection in a consecutive group of patients. All patients had VATS esophagectomy and 52.5% had had prior chemoradiotherapy. Given the patient population, a higher RLN injury rate may be expected. The incidence of right RLN injury was expectedly to be low, since the nerve is more constant in its position and the area that requires dissection is small. But for the left side, after chemoradiation nearly twice the rate of nerve palsy was found compared to those who had not although the numbers were still small to make this statistically significant. It is the author’s experience that after chemoradiation to the superior mediastinum, dissection in the left paratracheal area is more difficult. To strike a balance between striving for cure and increased morbidity, intraoperative decisions were made so that in some patients, RLN nodal dissection was not performed. In those that surgery was found to be palliative, there was no additional benefit of RLN dissection, with its attendant morbidity. Intraoperative difficulty of exposure (poor one-lung anesthesia) also hampered safe dissection in some.
Pre-operative preparation
All patients undergo routine workup and staging. Patient’s general fitness for surgery is assessed by cardiologist, anaesthetist and respiratory physician. Lung function test and echocardiogram are routine for every patient. Preoperative education on nutrition and perioperative breathing exercise are given. The surgeon responsible interviews patients and relatives on recovery milestones. Risks and operative procedure are properly explained. Disease staging methods include ultrasound of the neck for any cervical lymph nodes, endoscopic ultrasound, bronchoscopy and positron emission tomography/computer tomography scans (PET-CT). The management plan will be formulated in multidisciplinary meeting, consisting of surgeons, clinical oncologists, radiologists and pathologists. The potential positive nodes, especially at the RLN region were mapped out meticulously. For advanced tumour, we mainly adopted the CROSS regimen (weekly administration of carboplatin and paclitaxel with concurrent radiotherapy 41.4 Gy) for clinical T2–3 (bulky tumor) and N+ (including cervical lymphadenopathy) patients (8). All patients have preoperative documentation of bilateral vocal cord mobility before surgery.
Equipment preference card
For COIMM, an 8.0 mm single-lumen reinforced endotracheal tube with integrated electrodes for monitoring of laryngeal EMG is used (NIM Contact Reinforced EMG Endotracheal tube; Medtronic Inc., Jacksonville, FL, USA). CIONM works through continuous nerve monitoring equipment with APS (NIM-Response® 3.0 with the APS® Electrode; Medtronic Inc., Jacksonville, FL, USA). Intermittent nerve stimulation is used for RLN mapping (Ball-tip Monopolar Stimulator Probe; Medtronic Inc., Jacksonville, FL, USA).
Procedure
The operative technique is based on our standard MIE method as described previously (9) with several modifications. An 8.0 mm single-lumen reinforced endotracheal tube with integrated electrodes for monitoring of laryngeal EMG is used (NIM Contact Reinforced EMG Endotracheal tube; Medtronic Inc., Jacksonville, FL, USA). A single dose of short acting neuromuscular relaxant is given at induction of anesthesia and minimal neuromuscular blockade is used during the entire operation to ensure correct reading of the EMG. Before the availability of CIONM, our routine MIE starts with the VATS phase, after completion of the thoracic part of the operation the patient will be turned supine. Laparoscopic gastric mobilization will be performed, a left sided cervical incision (if formal bilateral neck dissection is not planned) is then made, the esophagus will be dissected out in the neck, divided and the esophagus delivered into the abdomen. The gastric conduit will be prepared extra-corporeally, followed by delivery to the neck for anastomosis to the esophageal remnant. With CIONM, in order to place the vagus nerve electrode, the operation starts with the cervical phase (Figure 1). The patient lies supine and the operation begins by dissecting and isolating the left vagus nerve for a short segment. A 2-mm nerve electrode is then clipped onto the nerve. The flange of the electrode is then secured to the carotid sheath by fine sutures. This makes it less likely for the electrode to dislodge when the patient is positioned in the left lateral position for the VATS phase. Just below the clavicle ground electrodes are placed. This completes the circuit for nerve monitoring. The neck wound is closed temporarily. At the author’s institution, the preferred method for one-lung ventilation is to use a right-sided bronchial blocker. The patient is turned to the left lateral position for VATS. Neuromuscular blockage may be necessary during repositioning to prevent excessive patient movement during repositioning. After turning to the left lateral position, the position of the endotracheal tube may have to be adjusted to ensure best contact between the electrodes and the vocal cords. The baseline EMG for CIONM is established 30 minutes after the last dose of muscle relaxant. A minimal stimulated EMG of 200 uV with 1mA stimulation is considered acceptable. From previous work on thyroidectomy, the manufacturer recommends the threshold for the alarm to be set to 50% reduction in EMG amplitude or >10% increase in latency (4). During esophagectomy both RLNs are at risk. Only the left RLN was monitored by COINM for two reasons. First the right RLN is easier to identify and there is less chance of injuring it because of its constant position looping around the subclavian artery and its length is also relatively short. The lymph nodes lie posteriorly to the nerve (Figure 2). On the other hand, the left RLN is much longer inside the mediastinum, the lymph nodes are often located anterior to the nerve in the left paratracheal space and therefore technically more difficult to dissect and the corresponding chance of injury high. Second, it is our practice to be selective in carrying out bilateral cervical nodal dissection; so most patients’ anastomoses are done in the left neck. Vagus nerve stimulation frequency is set to once per 10 seconds during VATS. The stimulation frequency is increased to once per second when dissection approaches the left RLN. Intermittent stimulation and mapping of both nerves using a long nerve probe is also used (Ball-tip Monopolar Stimulator Probe; Medtronic Inc., Jacksonville, FL, USA). Observing the CIONM signals (for the left RLN) as well as direct nerve stimulation with the ball probe before wound closure after VATS phase allows integrity of the nerves to be tested (Figure 3). The patient is then turned supine again for laparoscopic gastric mobilization. Before reopening the neck wound, a baseline for CIONM is re-established. CIONM is utilized during the dissection in the neck. The esophagus has usually been dissected out high up to the neck during the thoracic phase, so it is easily pulled out in the neck (Figure 4). It is then divided and the distal end pulled down to the abdomen. After making the gastric conduit, the stomach is delivered to the neck again esophago-gastrostomy. After completing the anastomosis, the vagus electrode is removed. Some of the sampled EMG tracings are shown (Figures 5,6).



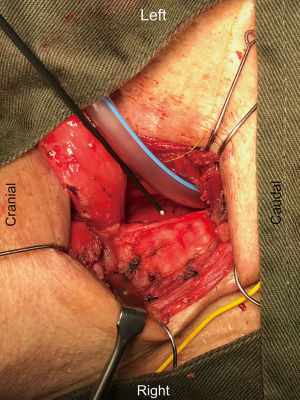
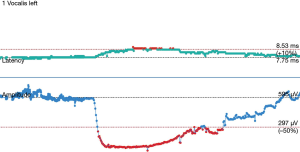
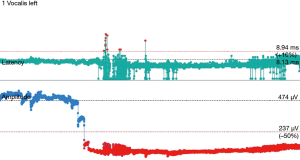
Role of team members
Close communication among surgeons, anaesthetists, scrub nurse and circulating staff is essential. For anaesthetists, as discussed above, the position between the electrodes on the endotracheal tube and the vocal cords affect tremendously on the baseline reading, and so does the timing and dosage of muscle relaxation. A good thoracoscopic view can only be achieved under perfect one-lung ventilation. Frequent adjustment of the bronchial blocker is needed due to the unpreventable dislodgement with intraoperative manipulations and retractions. For scrub nurse and circulating staff, they have to be accustomed to the wiring, connection and operation of the machine itself. Care has to be taken during the changing of position as it may cause dislodgement of the nerve electrode at the vagus nerve. Re-establishment of a baseline EMG is required at every stage of the operation. Familiarizing the procedure can make the whole operation being more efficient and safe.
Post-operative management
Extubation at the end of the operation is routine unless the operation is prolonged or complicated, in which case over-night ventilation is maintained. A flexible bronchoscopy with direct laryngoscopy would be performed the next morning for assessment of vocal cord mobility and function for all patients. Regular chest physiotherapy training is provided. Early mobilization is encouraged and oral diet is started on day 4 after surgery. Water soluble contrast study is not a routine. For patients with unilateral vocal cord palsy, we advocate early injection thyroplasty as early as in the first week, to reconstitute the function of airway protection. The patients are then followed up in outpatient clinic according to standard protocols.
Tips, tricks and pitfalls
For the technique of CIONM, after a short learning curve, it took around only 20 minutes for placement of the electrode on the left vagus nerve. Identification and placement of the electrode was usually straightforward; the longer time required for some patients was related to set up time of the machine and the need to wait for muscle relaxant to wear off before proper reading could be registered. This occurred early in the experience. Various techniques to ensure success in the placement and monitoring of the system include: (I) secure the electrode to the carotid sheath with 5/0 prolene suture; (II) putting a small gauze inside the neck wound before temporary closure to make it a compact space to lessen the chance of slippage of the electrode on the vagus nerve; (III) minimize movement of the neck when patients were turned to the left lateral position (± giving a small amount of muscle relaxant during the process to avoid excessive choking and airway movement); (IV) drop in the EMG after turning patient to the left lateral position was often related to displacement of the endotracheal tube itself with less than optimal apposition of the sensor with the vocalis muscles. Adjustment of the endotracheal tube will improve the EMG reading again; (V) resetting the EMG baseline after position change as well as each stage of the operation, especially just before left RLN nodal dissection, will ensure the readings were true reflection of the nerve function. Short episodes of minor reduction of EMG amplitude during dissection were encountered in many patients, usually caused by traction of tissue near the nerve. The EMG signal would usually revert to baseline with prompt release of traction. Intermittent nerve stimulation by the long probe helps to confirm course of the nerve and to test for integrity of nerve conduction. In some patients who developed palsy, the EMG gave immediate feedback but nerve injury has already occurred. This was particularly related to use of energy source next to the nerve or misidentification of the nerve before energy was applied. Use of COINM could not prevent such insults. In some other patients, traction injury happened despite COINM. Again, in these patients the traction force was probably too great before the surgeon could be alerted.
Discussions
Increasingly, the prognostic benefits of extended lymphadenectomy have been shown for both squamous cell as well as adenocarcinomas (13-19). For squamous cell carcinoma, there is a high incidence of nodal metastases along both RLN chains. Three-field lymphadenectomy, whereby extended nodal dissection is performed in the upper abdomen, mediastinum and bilateral neck has been advocated in Japan since the 1970s. Often the debate is mistakenly centered on whether additional bilateral cervical lymphadenectomy is needed; the real issue is the bilateral RLN chains, which extend from the mediastinum to the neck as a continuum, and this should more appropriately referred as the cervical-thoracic RLN chain. If mediastinal RLN nodal dissection is thorough, most of the paratracheal nodes in the “neck” have been harvested from the thoracic phase. The addition of “cervical lymphadenectomy” mainly involves more laterally situated nodes, for example in the supraclavicular fossa (level IV, V or station 104 according to the Japanese classification). Performing such extensive nodal dissection naturally will incur morbidity. RLN nerve injury will not only result in hoarseness of voice; poor cough effort will impair sputum expectoration in the postoperative period and increase chance of pulmonary complications. The less protected airway will predispose to aspiration pneumonia. Bilateral RLN palsies would even compromise airway patency and may necessitate tracheostomy. In the long-term, quality of life is worse. There is a balance between striving for nodal clearance by performing RLN nodal dissection, and the safety of this maneuver. Techniques to enhance the safety of RLN lymphadenectomy will be useful.
The incidence of nerve palsy reported in the literature is highly variable; the aggressiveness of RLN nodal dissection varies, from surgeons who do not perform RLN nodal dissection at all, to those who practiced three-field lymphadenectomy (both in the mediastinum as well as bilateral neck) as a routine. Naturally when the latter is performed, the RLN palsy rate will be high. In addition, the method of diagnosing RLN palsy is also important. A recent study showed that of 178 patients who had RLN palsy diagnosed on routine laryngoscopy, 26 (15%) did not have hoarseness of voice. Thus using hoarseness alone to diagnose RLN palsy will underestimate this complication. A review of three-field lymphadenectomy in Japan yielded an overall RLN palsy rate of 27% (20), some studies have shown up to 60% (1,21). In a recent detailed study on 299 patients, 60% developed RLN palsy, bilateral in 33%, right RLN in 8% and left in 59%. Various surgical approaches were used including transhiatal resection, and not all had RLN nodal dissection. In those with transthoracic or VATS resection, RLN palsy rate was 67% (1). The right RLN is much less prone to injury, as its anatomic course is more constant, and its length is short. The main purpose of using COINM therefore, is to aid left RLN dissection. With the availability of nerve mapping and CIONM, the author has become more aggressive in RLN nodal dissection. This is an interesting and important point to pursue further. In one study, Zhong and associates have reported increased number of involved mediastinal lymph node harvested and better 2-year survival rate in patients with nerve monitoring. At the same time less RLN paralysis and post-operative pneumonia were found. Conventional nerve monitoring technique without CIONM was used in the study (22). Having utilized this method, we feel that it will not be appropriate to conduct a study to compare nodal dissection with and without monitoring. What could be looked at in the future is to compare intermittent nerve probe mapping vs. CIONM. The safety, nodal harvesting, and cost benefit ratio can be investigated. In conclusion, we demonstrated the techniques and results of intermittent nerve mapping together with CIONM. Although clear evidence of its advantages could not be shown, we are confident that the learning curve has been overcome. Technical issues remain. Future project can aim to refine the method further, and to compare intermittent nerve mapping vs. CIONM to see if the technique will truly improve immediate as well as long-term outcome.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sato Y, Kosugi S, Aizawa N, et al. Risk Factors and Clinical Outcomes of Recurrent Laryngeal Nerve Paralysis After Esophagectomy for Thoracic Esophageal Carcinoma. World J Surg 2016;40:129-36. [Crossref] [PubMed]
- Tachibana M, Kinugasa S, Yoshimura H, et al. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg 2005;189:98-109. [Crossref] [PubMed]
- Ulmer C, Koch KP, Seimer A, et al. Real-time monitoring of the recurrent laryngeal nerve: an observational clinical trial. Surgery 2008;143:359-65. [Crossref] [PubMed]
- Schneider R, Randolph GW, Sekulla C, et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013;35:1591-8. [Crossref] [PubMed]
- Tsang RK, Law S. Adaptation of Continuous Intraoperative Vagus Nerve Stimulation for Monitoring of Recurrent Laryngeal Nerve During Minimally Invasive Esophagectomy. World J Surg 2016;40:137-41. [Crossref] [PubMed]
- Wong IY, Law S. Surgery in the era of neoadjuvant therapy for cancer of the esophagus. Esophagus 2016;13:105-9. [Crossref]
- Biere SS, Maas KW, Bonavina L, et al. Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial). BMC Surg 2011;11:2. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Law S. Esophagogastrectomy for carcinoma of the esophagus. In: Fischer J. editor. Mastery of Surgery. 6th ed. Philadelphia: Wolters Kluweer, Lippincott Williams & Wilkins, 2012:886-903.
- Wong I, Tong DK, Law S, et al. Intubation by reinforced endotracheal tube with integrated electrodes. Isolation of the left vagus nerve for clipping of 2 mm nerve electrode. Asvide 2017;4:023. Available online: http://www.asvide.com/articles/1329
- Wong I, Tong DK, Law S, et al. Right recurrent laryngeal nerve dissections. Ball-tip Monopolar Stimulator Probe was used for intermittent recurrent laryngeal nerve mapping. Asvide 2017;4:024. Available online: http://www.asvide.com/articles/1330
- Wong I, Tong DK, Law S, et al. Left recurrent laryngeal nerve dissection. Both CIONM & Ball-tip Monopolar Stimulator Probe were used for recurrent laryngeal nerve dissection. Asvide 2017;4:025. Available online: http://www.asvide.com/articles/1331
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248:979-85. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012;106:742-7. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72; discussion 972-4. [Crossref] [PubMed]
- Tachibana M, Kinugasa S, Yoshimura H, et al. Extended esophagectomy with 3-field lymph node dissection for esophageal cancer. Arch Surg 2003;138:1383-9; discussion 1390. [Crossref] [PubMed]
- Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 1998;175:47-51. [Crossref] [PubMed]
- Zhong D, Zhou Y, Li Y, et al. Intraoperative recurrent laryngeal nerve monitoring: a useful method for patients with esophageal cancer. Dis Esophagus 2014;27:444-51. [Crossref] [PubMed]
Cite this article as: Wong I, Tong DK, Tsang RK, Wong CL, Chan DK, Chan FS, Law S. Continuous intraoperative vagus nerve stimulation for monitoring of recurrent laryngeal nerve during minimally invasive esophagectomy. J Vis Surg 2017;3:9.


