Robotic esophagectomy
Introduction
The arrival of laparoscopic and thoracoscopic surgery in the 1980s paved the way for the first reported minimally invasive esophageal surgery in the 1990s. Minimally invasive esophagectomy (MIE), or MIE, refers to performing either or both the thoracic or abdominal portions of the case with laparoscopic or robotic assistance. Collard et al. first described esophageal resection by thoracoscopy in 1993 (1). This minimally-invasive approach documented shorter operative times, less blood loss, and shorter stays in the ICU with no increase in morbidity compared with the open approach (2). After the FDA’s approval of The Da Vinci robotic surgical system for use in laparoscopy in 2000, Melvin et al. became the first to report robotic esophagectomy in 2002 (3). Since Melvin’s pioneering operation, the use of robotic technology for esophagectomy has become increasingly common.
Indications
Indications for robotic esophagectomy parallel those of other MIE approaches: Barrett esophagus with high-grade dysplasia, end-stage achalasia, esophageal strictures, and esophageal cancer (4-8). While many T4 esophageal cancers are not amenable to surgical resection, selected patients have safely undergone en bloc resection of aorta or intrathoracic trachea or carina along with esophagectomy, but this would generally be a contraindication to robotic esophagectomy (9,10). Locally advanced cancers that are down-staged with neoadjuvant chemoradiotherapy are also amenable to robotic approach. Prior thoracic and abdominal surgery is not necessarily a contraindication but can certainly pose a greater challenge to the surgeon and should only be attempted in conjunction with the surgeon’s comfort level. Robotic esophagectomy may allow surgeons to consider resection on somewhat older and more comorbid patients, as there is evidence to support a decreased perioperative complication rate, specifically respiratory complications (11). Early stage cancers (T1a and superficial T1b) can be managed with endoscopic mucosal resection (EMR). If a lesion is not amenable to EMR or is T1b or deeper on final pathologic analysis, esophagectomy should be considered. If EMR is performed in the context of Barrett’s esophagus, radiofrequency ablation (RFA) to promote regression of the Barrett’s should also be considered. Patients with persistent high-grade dysplasia after attempted RFA should also be considered for esophagectomy.
Equipment
The Da Vinci Surgical System is currently the only FDA-approved robotic system for lung surgery. The surgeon sits at a console some distance from the patient who is positioned on an operating table in close proximity to the robotic unit with its four robotic arms. The robotic arms incorporate remote center technology, in which a fixed point in space is defined, and about it the surgical arms move so as to minimize stress on the thoracic or abdominal wall during manipulations. The small proprietary Endowrist instruments attached to the arms are capable of a wide range of high-precision movements. These are controlled by the surgeon’s hand movements, via ‘master’ instruments at the console. The ‘master’ instruments sense the surgeon’s hand movements and translate them electronically into scaled-down micro-movements to manipulate the small surgical instruments. Hand tremor is filtered out by a 6-Hz motion filter. The surgeon observes the operating field through console binoculars. The image comes from a manoeuvrable high-definition stereoscopic camera (endoscope) attached to one of the robot arms. The console also has foot pedals that allow the surgeon to engage and disengage different instrument arms, reposition the console ‘master’ controls without the instruments themselves moving, and activate electric cautery. A second optional console allows tandem surgery and training. Da Vinci currently offers both the Xi and Si systems. The Xi system is newer and features an overhead beam that permits rotation of the instrument arms, allowing for greater flexibility in terms of direction of approach of the robot to the patient. Compared to the Si, he Xi also has thinner instrument arms, longer instruments themselves, and the option to switch the camera to any arm/port.
Preoperative evaluation
The preoperative evaluation is no different for robotic esophagectomy than for open or other forms of MIE. A history and physical exam focused on elements such as gastroesophageal reflux disease, Barrett’s esophagus, achalasia and other motility disorders, prior surgeries, cardiac and pulmonary comorbidities, and functional status. Esophagoscopy should be performed to obtain the tissue diagnosis, rule out a synchronous secondary primary, as well as document location of tumor and presence of associated findings such as Barrett’s esophagus. Bronchoscopy is necessary in all proximal and middle-third tumors to evaluate local airway invasion or a synchronous second primary. Endoscopic ultrasound locally stages the tumor by evaluating the depth of penetration and involvement of regional lymph nodes, also offering fine-needle aspiration biopsy of suspicious lymph nodes if necessary. PET-CT of the chest, abdomen, and pelvis fulfills the staging for distant disease.
Assuming no metastatic disease is present, the patient’s cardiopulmonary function gets evaluated via pulmonary function and cardiac stress tests to ensure tolerance of single lung ventilation and carbon dioxide insufflation, which are typically employed during robotic esophagectomy. The patient’s physiologic status should be improved preoperatively if necessary. Smoking cessation should be encouraged and alcohol use documented in order to screen for cirrhosis and anticipate possible withdrawal sequelae in the post-operative period. Induction chemoradiation is instituted for patients with nodal disease or T2 or greater penetration of tumor as noted from EUS. For patients undergoing neoadjuvant therapy, correction of malnutrition before surgery can markedly reduce the morbidity and mortality before resection. Patients with esophageal cancer who are obstructed or experiencing dysphagia will likely need assistance with nutrition pre-operatively during neoadjuvant therapy. Compared with enteral feeding with a feeding jejunostomy, oral alimentation after placement of a covered silicone stent results in better relief of dysphagia, higher performance status, better tolerance of chemoradiotherapy, and better quality of life (12-14).
After completion of neoadjuvant chemoradiotherapy, restaging PET-CT should be performed to rule out progression of disease or metastasis. Patients who have persistent disease or show a complete or partial response based on the lesion’s FDG avidity on PET-CT are scheduled for esophagectomy 6–10 weeks after completion of neoadjuvant therapy. Lee et al. found that an increased time interval from completion of neoadjuvant therapy to surgery, while resulting in increased pathologic complete response rate, did not lead to an improved overall survival, and in fact, overall survival was worse when waiting greater than 64 days (15).
Patient positioning/port placement
Abdominal portion of procedure
The abdominal portion of the procedure is carried out via a robotic approach. The patient is placed in the supine position. At our institution, arterial and central venous lines are not typically used. A Foley catheter and nasogastric tube are placed, with special note to back all lines/tubes from the esophagus and stomach prior to stapler deployment. Both arms are tucked with foam padding at the elbow and wrist if body habitus allows. The patient should be secured to the operating room table with a large strap at the superior thigh and a foot board may be used to accommodate steep reverse Trendelenburg positioning.
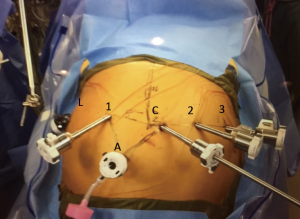
Access to the abdomen can be performed by whatever means is comfortable to the surgeon. We use a Hassan technique and initially place a 12-mm camera port 18 cm inferior to the xiphoid process. We use a 30 degree down robotic camera. Figure 1 shows the typical port placement for the abdominal phase of the operation. These should be spaced no more than 2–3 cm above the camera port and 9 cm apart. If the patients left side of the abdomen does not allow this due to space, the robotic arms may be staggered. If using the Xi system and robotic stapling is preferred, a 12-mm port should be placed for the left robotic arm. The two left upper quadrant ports should be 8-mm ports (if using Si system the 2nd port can be a 5-mm port). A 5-mm port is placed as close to the costal margin and laterally as possible to accommodate a liver retractor. We use a Snowden Pencer articulating pretzel retractor (Becton Dickinson; Franklin Lakes, NJ, USA). A 12-mm assistant port is placed in the patient’s right lower quadrant, triangulated behind the left robotic arm port and camera port. This port is also used to deliver insufflation.
The preferred instrument selection is as follows: left robotic arm—Cadiere forceps, right robotic arm—vessel sealer, second right robotic arm- thoracic grasper (Si system) or tip-up fenestrated grasper (Xi system). If using the Si system, the operating room table is turned such that the robot can drive in over the patients head. The Xi system does not require the bed to be turned.
Thoracic portion of the procedure
At the completion of the abdominal portion of the procedure, while the patient is supine, a double lumen endotracheal tube is placed. The patient is then placed in the left lateral decubitus position. We do not use a bean bag for stabilization, but rather pad the patient with blankets/foam and secure the patient with cloth tape. A forward lean is desired to allow ideal access to the posterior mediastinum. The right lung is then excluded.
Figure 2 shows the port placement for the thoracic phase of the operation. The first port placed is the camera port which is placed 9 cm inferior and slightly posterior to robotic arm 1. Robotic arm 1 is placed just below the hair line of the right axilla in the anterior axillary line. A 5-mm port is initially used as the camera port (upsized to 8-mm or 12-mm for Xi and Si, respectively). A 5-mm 0 degree thoracoscopic camera is then inserted into the camera port to evaluate the pleural space for intra-thoracic adhesions and to visualize further port placement. Insufflation of warm carbon dioxide is carried out to 12 mmHg which compresses the lung and lowers the diaphragm. It is important to carefully plan all four robotic port sites as this will minimize instrument collisions and limitations. We suggest using a ruler and marker to plan insertion sites. An 8-mm port for robotic arm 1 is then placed at the previously mentioned site. Robotic arm 1 serves as the surgeon’s right hand. The next 8-mm port placed is for robotic arm 2. This incision is made 9 cm inferior to the camera port and slightly more posterior, tracking toward the right hip. Robotic arm 3 is a 5-mm port that is positioned 10 cm from robotic arm 2 as far posterior and inferior as tolerated. The final port placed is a 12-mm assistant port. This trocar should be triangulated with the camera and robotic arm 2 trocar anteriorly. The insufflation should be changed to this port as to not interfere with the robotic arms.
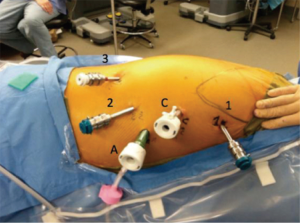
The preferred instrument selection is as follows: robotic arm 1 (right hand)—thoracic dissector with bipolar energy, robotic arm 2 (left hand)—Cadiere forceps, robotic arm 3—thoracic grasper (Si) or tip-up fenestrated grasper (Xi).
Conduct of operation
Abdominal portion of the procedure
The greater omentum is divided from the greater curve of the stomach by using the vessel sealer on the left side of the abdomen. The dissection is carried from the patients left to right until the pylorus is reached. Special caution is made to avoid injury to the right gastroepiploic artery. Attention is then turned to the short gastric arteries and continuing the dissection to the fundus. An omental flap should be preserved to be later used to wrap the anastomosis and protect the airway. The 2nd right robotic arm should be used to hold the colon/omentum in one direction while the assistant can hold gentle retraction on the stomach. Once the short gastric arteries are divided the retroperitoneal stomach attachments should be divided and the left side of the esophageal hiatus should be mobilized. The area beneath the esophagus should be as clear as possible to later facilitate encircling of the esophagogastric junction. The lesser omentum is then incised. The left gastric artery should be carefully inspected for an accessory or replaced left hepatic artery. Up to 12% of patients may have this anatomic variation (16). If encountered a clip may be placed and viability of the liver may be assessed. The left gastric artery is then ligated with a vascular stapler. Dissection is performed circumferentially around the esophagus and a few centimeters into the mediastinum. A 1 cm thick Penrose is then circumferentially placed around the esophagus and the ends secured together.
Botulinum toxin injection is then performed on the pylorus (Figure 3). We use 100 units in 4 mL of saline. Depending on surgeon discretion, pyloromyotomy or pyloroplasty may alternatively be performed. The pylorus should be able to reach the hiatus with minimal tension. The gastric conduit is then constructed using a linear stapler. We use a 4-mm staple height (45–60 mm length). The stomach is retracted laterally by the 2nd right robotic arm on the fundus and the assistant retracting the antrum. The stomach is not completely transected so that the specimen and conduit may be pulled into the chest. We typically place a suture in the distal portion of the staple line to easily identify it in the chest. A jejunostomy tube can be placed laparoscopically at this time if not placed preoperatively.
Thoracic portion of the procedure
With the bipolar thoracic dissector in robotic arm 1 (right hand) the mediastinal pleura is incised. Robotic arm 3 (2nd left hand) can retract the lung for exposure. The pleura are opened from the azygous vein down to the diaphragm (Figure 4). The inferior pulmonary ligament and associated nodal tissues are dissected free. The esophagus is dissected away from the aorta with special care to achieve hemostasis from perforating branches (Figure 5). This is typically done with bipolar energy and not clips. All paraesophageal tissue, including lymph nodes, should be dissected free from the diaphragm to thoracic inlet (Figure 6). Special considerations include harvesting subcarinal nodes, nodes adjacent to left and right main stem bronchi and careful attention to avoid thermal injury to the airway. Thermal injury may present as esophagobronchial fistula and can cause significant morbidity for the patient. The azygous vein is transected with a vascular staple load (Figure 7). We recommend transection as posterior as possible to avoid a long stump that may obstruct anastomosis creation.
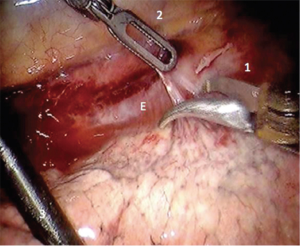
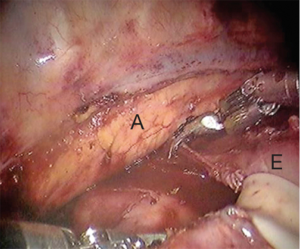
Using the Penrose that was placed during the abdominal portion of the procedure the distal esophagus and gastric conduit are delivered into the chest. Bipolar scissors are then placed in robotic arm 1. While pulling distal traction the proximal esophagus is divided just above the azygous vein. The Cadiere forcep is then placed in robotic arm 1 and the assistant delivers the conduit further into the thoracic cavity. A stapler is then used to divide the specimen from the gastric conduit. The stapled edge of the conduit should be oriented laterally.
Silk sutures are then placed anteriorly and posteriorly from the conduit to the pleura to reduce tension on the anastomosis and maintain orientation.
Hand-sewn technique
In preparation of creating a double-layered esophagogastrostomy, atraumatic forceps are placed in robotic arm 2 and a suture-cut needle driver in robotic arm 1. Special note should be made to place the anastomosis as far away from the staple line towards the greater curve as possible. A row of interrupted 3–0 silk suture (10 cm long) is placed in the seromuscular layer along the “back wall” of the anastomosis. With the electrocautery a transverse gastrotomy is then made 2–3 cm in diameter on the posterior surface of the stomach and 5 mm away from the previously placed silk suture line (Figure 8). A 3–0 Vicryl is then placed at each corner of the anastomosis. Full-thickness purchases are then made in a running fashion performing the “back wall” first. Prior to completion of the “front wall” the nasogastric tube is passed into the conduit under direct visualization. An additional row of 3–0 silk sutures are placed in an interrupted fashion on the “front wall” to complete the two layer anastomosis. If possible a piece of omentum is then delivered from the abdomen and buttressed between the conduit and airway and also covering the anastomosis. A suture is then placed at the right hemidiaphragm to the conduit to avoid herniation of abdominal contents into the chest. A hybrid technique involving stapling of the “posterior” wall of the anastomosis and hand-sewn “anterior” wall can also be performed (Figure 9).


Mechanical stapler technique
A purse string is created in the proximal esophagus. A 3–0 non-absorbable monofilament suture is used with special care to incorporate the mucosal layer. The anvil of the EEA stapler is placed in the esophagus and the purse string is tied. An additional purse string layer may be placed if there is concern for gaps around the anvil. A gastrotomy is then performed at the tip of the conduit. The EEA stapler is then placed through the gastrotomy. The tip of the stapler is then deployed through the superior/posterior wall of the conduit. Special care should be made to avoid multiple passes of the tip through the conduit and deployment of the tip into the aorta. The anvil and stapler tip and then connected and the stapler is fired. Careful inspection of the tissue in the stapler should show two complete rings of tissue. A linear stapler can then be used to close the gastrotomy.
A large specimen retrieval bag is then placed via the assistant port and the specimen is removed. One chest tube is placed posteriorly and toward the apex via robotic arm 2 port site. The ports are removed and insufflation discontinued, while inspecting port sites for bleeding. The lung is then re-expanded under direct visualization. The port sites are then closed (18).
Pearls/pitfalls
- The patient should be nutritionally optimized. Consider preoperative jejunostomy tube placement if the patient is losing weight or unable to maintain nutritional goals;
- Preparation and appropriate planning of trocar placement is key to avoid instrument collisions and frustration in robot docking;
- Be mindful of aberrant arterial anatomy. If there is concern for replaced left hepatic artery occlude the vessel and assess liver viability prior to ligation;
- Delivery of the gastric conduit into the chest should be performed by the assistant using a non-traumatic instrument (i.e., sponge forcep). Due to lack of haptic feedback on the robotic instruments, excessive force or traumatic handling of conduit is possible;
- Avoid monopolar electrocautery near the left/right mainstem bronchus. Unrecognized airway injury can lead to esophagobronchial fistula, a devastating complication.
Results
MIE with thoracoscopic assistance has been shown to be a safe and effective modality for esophageal resection (7). Biere and colleagues showed that VATS can reduce pulmonary complications after esophagectomy when compared to thoracotomy in a randomized controlled trial (19). The first series of robotic resections were performed by Kernstine et al. Fourteen patients were included in this series with good results. Anastomosis in this series was performed in the neck (20). Retrospective analysis by Weksler and colleagues showed that robotic esophagectomy was equivalent to thoracoscopic approach. Forty-three patients were reviewed and no difference was found in operative time, blood loss, number of lymph nodes resected, postoperative complications, days of mechanical ventilation, lengths of ICU stay or lengths of overall hospital stay (21). Survival data comparing open to thoracoscopic resection has historically been equivalent (22). As with the thoracoscopic approach, long term robotic survival data does not exist. In regard to anastomosis technique, no difference is noted between hand-sewn versus mechanical stapler technique, but there has been a trend toward increased stricture rate using the stapler technique (23,24).
In 2013 we reported our institutional experience for patients undergoing robotic esophagectomy with intra-thoracic anastomosis. Twenty-two patients underwent resection with no 30- or 90-day mortalities. Only three patients experienced minor morbidity which was related to urinary retention or atrial fibrillation. No patients underwent conversion to thoracotomy and only one patient required conversion from laparoscopy to laparotomy due to staple line breakdown. The median number of lymph nodes removed was 18 (range, 15–26). All patients received a pathologic complete (R0) resection (25). All 22 patients were alive at short-term (5 months) follow up and were without recurrence of disease. It is the author’s beliefs that the robotic approach provides optimal visualization with a high-definition stereoscopic surgeon controlled camera, superior lymphadenectomy and a medium that is applicable to the open surgeon.
Conclusions
Robotic esophagectomy is a safe procedure that offers outcomes equivalent to thoracoscopic and open resection. Potential benefits of improved optics and lymph node dissection have yet to be determined, but are potential advantages of robotic resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: R Cerfolio is a proctor and teacher for intuitive. And other authors have no conflicts of interest to declare.
References
- Collard JM. As originally published in 1993: En bloc and standard esophagectomies by thoracoscopy. Updated in 1996. Ann Thorac Surg 1996;61:769-70. [Crossref] [PubMed]
- Nguyen NT, Follette DM, Wolfe BM, et al. Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg 2000;135:920-5. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery. Initial experience in foregut surgery. Surg Endosc 2002;16:1790-2. [Crossref] [PubMed]
- Pierre AF, Luketich JD. Technique and role of minimally invasive esophagectomy for premalignant and malignant diseases of the esophagus. Surg Oncol Clin N Am 2002;11:337-50. x. [Crossref] [PubMed]
- Nguyen NT, Schauer P, Luketich JD. Minimally invasive esophagectomy for Barrett's esophagus with high-grade dysplasia. Surgery 2000;127:284-90. [Crossref] [PubMed]
- Luketich JD, Nguyen NT, Weigel T, et al. Minimally invasive approach to esophagectomy. JSLS 1998;2:243-7. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- Fernando HC, Luketich JD, Buenaventura PO, et al. Outcomes of minimally invasive esophagectomy (MIE) for high-grade dysplasia of the esophagus. Eur J Cardiothorac Surg 2002;22:1-6. [Crossref] [PubMed]
- Cong Z, Diao Q, Yi J, et al. Esophagectomy combined with aortic segment replacement for esophageal cancer invading the aorta. Ann Thorac Surg 2014;97:460-6. [Crossref] [PubMed]
- Van Raemdonck D, Van Cutsem E, Menten J, et al. Induction therapy for clinical T4 oesophageal carcinoma; a plea for continued surgical exploration. Eur J Cardiothorac Surg 1997;11:828-37. [Crossref] [PubMed]
- Li J, Shen Y, Tan L, et al. Is minimally invasive esophagectomy beneficial to elderly patients with esophageal cancer? Surg Endosc 2015;29:925-30. [Crossref] [PubMed]
- Brown RE, Abbas AE, Ellis S, et al. A prospective phase II evaluation of esophageal stenting for neoadjuvant therapy for esophageal cancer: optimal performance and surgical safety. J Am Coll Surg 2011;212:582-8; discussion 588-9. [Crossref] [PubMed]
- Bower M, Jones W, Vessels B, et al. Nutritional support with endoluminal stenting during neoadjuvant therapy for esophageal malignancy. Ann Surg Oncol 2009;16:3161-8. [Crossref] [PubMed]
- Siddiqui AA, Glynn C, Loren D, et al. Self-expanding plastic esophageal stents versus jejunostomy tubes for the maintenance of nutrition during neoadjuvant chemoradiation therapy in patients with esophageal cancer: a retrospective study. Dis Esophagus 2009;22:216-22. [Crossref] [PubMed]
- Lee A, Wong AT, Schwartz D, et al. Is There a Benefit to Prolonging the Interval Between Neoadjuvant Chemoradiation and Esophagectomy in Esophageal Cancer? Ann Thorac Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50-2. [Crossref] [PubMed]
- Broussard B, Evans J, Cerfolio R, et al. Hybrid anastomotic technique for Ivor Lewis esophagectomy. Asvide 2016;3:330. Available online: http://www.asvide.com/articles/1098
- Wei B, Cerfolio RJ, Hawn MT. Minimally Invasive Esophagectomy. In: Mulholland MW, Albo D, Dalman R, et al. Operative Techniques in Surgery. 1st ed. Philadelphia, PA: LWW, 2014:246-53.
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Shamoun DM, et al. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc 2007;21:2285-92. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Thomson IG, Smithers BM, Gotley DC, et al. Thoracoscopic-assisted esophagectomy for esophageal cancer: analysis of patterns and prognostic factors for recurrence. Ann Surg 2010;252:281-91. [Crossref] [PubMed]
- Honda M, Kuriyama A, Noma H, et al. Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg 2013;257:238-48. [Crossref] [PubMed]
- Markar SR, Arya S, Karthikesalingam A, et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol 2013;20:4274-81. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
Cite this article as: Broussard B, Evans J, Wei B, Cerfolio R. Robotic esophagectomy. J Vis Surg 2016;2:139.