Selecting incision-dominant cases for robotic liver resection: towards outpatient hepatectomy with rapid recovery
Introduction
Liver resection has evolved greatly over the last three decades. Once an operation with very high risk of hemorrhage and high mortality rate (1), most hepatectomies are now performed at major centers with a mortality rate routinely less than 3% (2). Transfusion is required in only a minority of patients (3). Techniques of liver resection have also evolved greatly. Minimally invasive hepatectomy was once discouraged. Recent advancements in equipment, technique, and training have resulted in general acceptance of laparoscopic hepatectomy (4). A number of studies have shown a trend for earlier discharge and adequate oncologic outcomes with laparoscopic hepatectomy (4), though clear superiority of the technique in objective outcome is sparse because of difficulties in measuring recovery outcomes in general.
Robotic hepatectomy is the latest evolution of minimally invasive surgery (MIS). With 3D optics, wristed instruments, integrated stapling, and sealing instruments, many tasks that were technical challenges for laparoscopic surgery are overcome including suturing and fine vascular dissection. These technical advantages have come at a higher monetary cost (5,6), sufficient to demand a justification by data on outcome to show advantage for patients. Most early robotic hepatectomy papers (7,8) had a high percentage of major liver resections (>35%), and touted as advantage of robotic hepatectomy the low conversions rate. In one paper (7), the advantage was cited as “more likely to complete operation in totally minimally invasive approach”.
In the current paper we proposed a different philosophy for robotic hepatectomy. We propose that the best operations to be performed robotically are those involving removal of small amounts of parenchyma, particularly for lesions placed in parts of the liver hard to reach by laparoscopy. For these poorly placed tumors, usually a large incision is required to resect tumor. We propose that it is for these “incision-dominant” operations, where the facial and muscle incision and not the physiology of liver regeneration dominate recovery, that robotic hepatectomy is most likely to help patients and be measured to do so. Following is our preliminary series demonstrating many of the advantages of such an approach.
Methods
Patients
This is an observational study of a prospectively maintained liver resection database. We included all patients 18 years or older, who underwent exploration with intent to perform a robotically-assisted liver resection. This paper includes patients submitted to robotic resection by the senior author (Yuman Fong) at the Memorial Sloan-Kettering Cancer Center, and operations performed by all surgical authors at the City of Hope Medical Center (Yuman Fong, Susanne Warner, Laleh Melstrom, Gagandeep Singh, Byrne Lee, Yanghee Woo). We excluded patients undergoing liver biopsy. The study was approved by the City of Hope Institutional Review Board #15097.
Data for these patients were extracted from the database and additional data relevant to the study were extracted from the electronic medical records. Data examined included demographics (age, gender, body mass index, American Society of Anesthesiologists classification, Eastern Cooperative Oncology Group performance status, comorbidities), indications and perioperative variables (extent of liver resection, estimated blood loss, duration of surgery, concomitant procedures). The size of liver resection was defined using the International Hepato-Pancreato-Biliary Association Couinaud’s classification (9). Major hepatectomy was thus defined as a resection of 3 or more segments. Margins were defined as positive (<1 mm), close (1–10 mm), and negative (>10 mm).
Complications and readmissions
All complications during the index hospitalization and readmission were recorded up to 90 days after surgery. Mortality was recorded at 90 days. Readmissions within 30 days after discharge from index hospitalization per the CMS definition were recorded. Each complication was categorized according to the Clavien-Dindo complication grading system as detailed elsewhere (10). Briefly, complications were graded from 1 to 5, where 1 and 2 usually indicate management with either oral or intravenous pharmacologic treatment; and where grade 3 indicates procedures requiring involvement of interventional radiology or gastroenterology, or return to the operating room. Grade 4 indicates treatment in the intensive care unit for organ dysfunction or permanent disability as sequelae of the complication; grade 5 indicates patient death.
Robotic operations
Patients were evaluated for suitability of the robotic approach depending on the location of their tumors, the quality of their liver, and the patient’s clinical status. Contraindications for robotic resection were invasion of the inferior vena cava (IVC) or base of hepatic veins close to the IVC, invasion of the main right or left portal vein, and need for vascular reconstruction or bile duct resection.
The Si da Vinci Surgical System or the Xi da Vinci Surgical System was used with four arms. Operative procedure was as described before (11). Patients were secured to a table with a footboard that allowed for 30 degrees of reverse trendelenburg. For the Si system, a 12-mm trocar was used to place a camera and three 8-mm ports were used for the instrument arms. Liver parenchyma was divided with a combination of harmonic scalpel, the vessel sealer, the PK bipolar dissector, or the stapler. The patient cart was always placed by the patient’s feet. The scrub nurse was positioned by the patient’s right leg, and the assistant surgeon or surgical physician assistant was positioned by the patients left leg.
Statistical analysis
Bivariate analysis of categorical variables was performed using the Chi-square test. Criterion for statistical significance set at P<0.05.
Results
Demographics
The study population consisted of 97 patients subjected to exploration for possible robotically assisted hepatectomy. Median age was 62 years with range 40–91 (Table 1). Fifty four percent were male. The diagnosis associated with these operations included metastatic tumors to the liver (65%), primary hepatocellular carcinoma (10%), biliary cancers (11%), and benign tumors and conditions (14%).
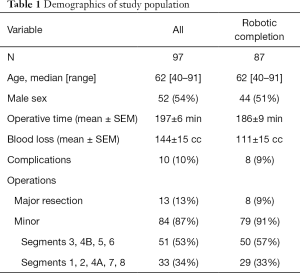
Full table
Conversion to open surgical procedure occurred in ten patients, mostly due to more extensive disease found. For the 87 procedures completed robotically, the mean operative time was 186±9 (median 170, range, 30–486) min. Mean blood loss was 111±15 (median 50, range, 25–800) mL. Complications occurred in 8 individuals (9%), and included two cases of pneumonia, two intra-abdominal abscesses, ileus, cellulitis, and urinary retention. There were no perioperative deaths.
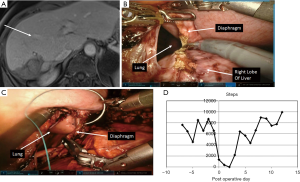
Procedures performed included eight major hepatectomies: two right hepatic lobectomies (one with in continuity diaphragmatic resection, Figure 1), and six left hepatic lobectomies. The majority of procedures were minor hepatectomies (91%), 57% of which involved the lower segments of liver (3, 4B, 5, 6), and 33% involving superior segments (1, 2, 4A, 7, 8). Minor procedures included 13 left lateral sectorectomies, and seven right posterior sectorectomies.
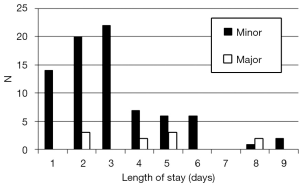
Hospital stay
Two thirds of the patients remained in hospital 3 days or less, including three patients subjected to hemihepatectomy (2 left and 1 right) (Figure 2). The three patients subjected to minor hepatectomies who stayed beyond 1 week in hospital were all over 80 years of age and stayed due to placement issues.
The strongest predictors of long hospital stay (>3 days) were major hepatectomy, complications, operative time >210 min, and blood loss >100 cc (Table 2).
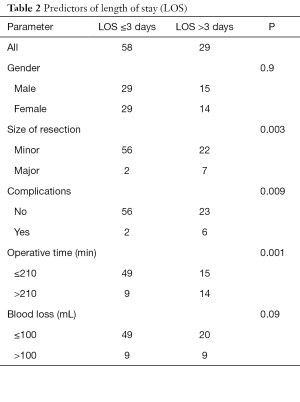
Full table
Fitness Sensor Tracking
For the patient subjected to right hepatic lobectomy and in-continuity diaphragmatic resection (Figure 1), we tracked her preoperative activity and post-operative recovery with a fitness tracker (Vivofit®, Garmin Ltd., Schaffhausen, Switzerland). The activity curve is shown in Figure 1D. Her level of activity was approximately 6,000 steps prior to surgery and recovered to 6,000 steps by day 4. She was discharged on day 2 post-operatively.
Discussion
Recovery from hepatectomy includes overcoming (I) surgical stress, (II) systemic effects of liver regeneration, (III) incisional discomfort, and (IV) associated ileus. MIS, including robotically-assisted MIS surgery is intended mainly to alleviate discomfort associated with a large incision and decrease deleterious effects associated with evaporate losses during a laparotomy. The current paper suggests that the patient most likely to benefit from robotically-assisted MIS hepatectomy is that patient subjected to an incision-dominant procedure. In particular, for minor hepatectomies in regions of the liver poorly accessible to laparoscopy (4), that normally would require large open incisions, robotic MIS hepatectomy offers an attractive solution with rapid recovery.
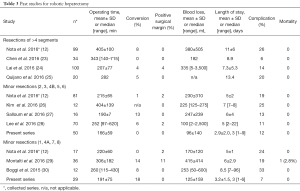
Robotic MIS hepatectomy as a field is moving past its infancy. Nota et al. (12) recently reported a collected series analysis of the 12 best studies up to 2015 (7,8,13-22). There is no doubt that hemi-hepatectomies can be performed with safety by the robotically-assisted approach (7,8). However, for patients requiring major hepatectomy, particularly operations that are long and requiring abundant fluid administration and blood transfusion, the recovery is likely to be dominated by liver regeneration or by surgical trauma. Consequently, operative times are long and hospital stays are very similar to that of open hepatectomy (Table 3). In these operations where recovery is dominated by the liver regenerative process, it will be difficult to prove superiority of a robotic approach, especially since the robotic approach will at least in the short term be more costly (5,6). Major hepatectomy will remain an operation performed by experts. The choice of whether to perform these robotically will be according to philosophies of individual centers. We have chosen to be highly selective in robotic hemi-hepatectomies, restricting these operations to cases where early discharge is likely: cases involving young, fit, motivated patients, with tumors away from the hilus or confluence of the hepatic veins.
Full table
The published data on robotically-assisted hepatectomy should be segregated into three groups: (I) major hepatectomy, (II) minor inferior hepatectomy, and (III) minor superior/posterior hepatectomy (Table 3). Minor inferior hepatectomy (segment 3, 4B, 5, 6, or left lateral sectorectomies) are procedures also suited for traditional laparoscopic surgery (4), since these are regions of the liver approachable by straight laparoscopic instruments. At present, either a laparoscopic or a robotic approach can be used to deliver the benefits of MIS surgery for patients with tumors in these parts of the liver (26), and traditional laparoscopic approach is less expensive (5,6). We predict however, that as 3D, high definition laparoscopy, and other high priced technology gain penetrance in laparoscopy, and as other robotic platforms reach market, routine laparoscopy and robotically-assisted MIS will approach price equivalency. The robotic approach has also proven to provide a low barrier for adoption of MIS surgery. As availability of robots increases worldwide, and as cost of robotic operations decrease, it is more likely that future surgeons and current surgeons dedicated to open operations will more likely learn the robotic approach than routine laparoscopic.
Minor superior-posterior hepatectomies (segment 1, 2, 4A, 7, 8, or right posterior sectorectomies) consist of operations thought poorly approachable by traditional laparoscopy (4). This is because it is difficult to reach these areas and performed skilled moves with straight instruments. Thus, tumors in these parts of the liver currently are approached by large open incisions, even when the tumors are small. If a randomized trial is desired for operations in superior, posterior segments, the randomization has to be against open operation. Other investigators have also recognized the advantages of a robotic approach for tumors in these regions (12,29). The articulated instruments and the 3D optics of the surgical robot are well suited for operations in these anatomically “remote” areas of the liver. The current study confirms that hepatectomy for tumors in these regions of the liver to be ideal for robotic MIS approach, achieving very low complication rates and very short hospital stays.
Measures of quality of hepatectomy and major surgery in general have traditionally relied on mortality and complication rates. In recent years, the mortality from hepatectomy at major center have approached 0–1% (31), and complication rate for minor hepatectomies are now routinely less than 20%. Surrogate markers for quality, such as blood loss, ICU stay, hospital stay, or re-admission to hospital are now used to stratify quality of surgical program. However, with improving surgical and anesthesia techniques, hemorrhage is a rare problem, and many “bloodless” hepatectomy (32) series are being reported with little use for transfusion. In the current study we show that good patient selection can lead to low blood loss, low complication and short stay for robotically assisted hepatectomy.
Ultimately, MIS surgery should be measured by functional recovery, since the biggest difference from open surgery is the size of the muscle and facial incision. Many have attempted to use (33) quality of life instruments to document the superiority of the MIS approach, but have been hindered by the how cumbersome some instruments are and the limited number of time points that can be accessed. We have begun a program evaluating use of athletic fitness devices to measure functional recovery (Figure 1D). We recently presented our pilot study demonstrating feasibility and acceptability of this approach (34). We are now focusing a study of such post-operative fitness tracking for MIS surgery.
Until recently, the idea that hepatectomy could in a major subset of patients be an outpatient procedure would have been unthinkable. Not long ago, papers reporting hepatectomy outcomes talked about length of ICU stay, and hospital stays of two weeks were routine. The current paper is one of the first reporting feasibility of outpatient or short stay hepatectomy. The results of the current paper indicate that selecting patients who are medically fit and who have physiologically favorable lesions (small parenchymal resection) and performing a short operation (to minimize surgical stress), and with small fascial and muscular incisions, can result in outpatients or short stay hepatectomy. We have also recently published our enhanced recovery after surgery (ERAS) pathways for hepatectomy, which includes a pathway for robotically-assisted hepatectomy (35).
Conclusions
Surgical outcomes are optimized when the surgeon (I) selects patients for the surgical approach most beneficial, and (II) performs the operation with skill. Selecting incision dominant procedures ensures highest likelihood for benefit from a robotic MIS approach. Robotically-assisted MIS surgery brings advanced tools to complement the skills of hepatic surgeons to further enhance outcomes in these difficult operations.
Acknowledgements
Funding: Supported in part by the Pilot Grant Program of the City of Hope Medical Center (P30 CA33572, NIH 5K12CA001727-20).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of City of Hope (No. 15097).
References
- Blumgart LH, Fong Y. Surgical options in the treatment of hepatic metastasis from colorectal cancer. Curr Probl Surg 1995;32:333-421. [Crossref] [PubMed]
- Park J, Chen YJ, Lu WP, et al. The evolution of liver-directed treatments for hepatic colorectal metastases. Oncology (Williston Park) 2014;28:991-1003. [PubMed]
- Kooby DA, Stockman J, Ben Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860-9. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Patti JC, Ore AS, Barrows C, et al. Value-based assessment of robotic pancreas and liver surgery. Hepatobiliary Surg Nutr 2017;6:246-57. [Crossref] [PubMed]
- Sham JG, Richards MK, Seo YD, et al. Efficacy and cost of robotic hepatectomy: is the robot cost-prohibitive? J Robot Surg 2016;10:307-13. [Crossref] [PubMed]
- Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: results for 70 resections. Surgery 2011;149:29-39. [Crossref] [PubMed]
- Couinaud C. Surgical anatomy of the liver Revisited. Paris: C. Couinaud, 1989.
- Clavien PA, Strasberg SM. Severity grading of surgical complications. Ann Surg 2009;250:197-8. [Crossref] [PubMed]
- Kingham TP, Leung U, Kuk D, et al. Robotic Liver Resection: A Case-Matched Comparison. World J Surg 2016;40:1422-8. [Crossref] [PubMed]
- Nota CL, Rinkes IH, Molenaar IQ, et al. Robot-assisted laparoscopic liver resection: a systematic review and pooled analysis of minor and major hepatectomies. HPB (Oxford) 2016;18:113-20. [Crossref] [PubMed]
- Wu YM, Hu RH, Lai HS, et al. Robotic-assisted minimally invasive liver resection. Asian J Surg 2014;37:53-7. [Crossref] [PubMed]
- Berber E, Akyildiz HY, Aucejo F, et al. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford) 2010;12:583-6. [Crossref] [PubMed]
- Choi GH, Choi SH, Kim SH, et al. Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc 2012;26:2247-58. [Crossref] [PubMed]
- Ji WB, Wang HG, Zhao ZM, et al. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg 2011;253:342-8. [Crossref] [PubMed]
- Kandil E, Noureldine SI, Saggi B, et al. Robotic liver resection: initial experience with three-arm robotic and single-port robotic technique. JSLS 2013;17:56-62. [Crossref] [PubMed]
- Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg 2013;205:697-702. [Crossref] [PubMed]
- Spampinato MG, Coratti A, Bianco L, et al. Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc 2014;28:2973-9. [Crossref] [PubMed]
- Troisi RI, Patriti A, Montalti R, et al. Robot assistance in liver surgery: a real advantage over a fully laparoscopic approach? Results of a comparative bi-institutional analysis. Int J Med Robot 2013;9:160-6. [Crossref] [PubMed]
- Felli E, Santoro R, Colasanti M, et al. Robotic liver surgery: preliminary experience in a tertiary hepato-biliary unit. Updates Surg 2015;67:27-32. [Crossref] [PubMed]
- Yu YD, Kim KH, Jung DH, et al. Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbecks Arch Surg 2014;399:1039-45. [Crossref] [PubMed]
- Chen PD, Wu CY, Hu RH, et al. Robotic Versus Open Hepatectomy for Hepatocellular Carcinoma: A Matched Comparison. Ann Surg Oncol 2017;24:1021-8. [Crossref] [PubMed]
- Lai EC, Tang CN. Long-term Survival Analysis of Robotic Versus Conventional Laparoscopic Hepatectomy for Hepatocellular Carcinoma: A Comparative Study. Surg Laparosc Endosc Percutan Tech 2016;26:162-6. [Crossref] [PubMed]
- Quijano Y, Vicente E, Ielpo B, et al. Robotic Liver Surgery: Early Experience From a Single Surgical Center. Surg Laparosc Endosc Percutan Tech 2016;26:66-71. [Crossref] [PubMed]
- Kim JK, Park JS, Han DH, et al. Robotic versus laparoscopic left lateral sectionectomy of liver. Surg Endosc 2016;30:4756-64. [Crossref] [PubMed]
- Salloum C, Lim C, Lahat E, et al. Robotic-Assisted Versus Laparoscopic Left Lateral Sectionectomy: Analysis of Surgical Outcomes and Costs by a Propensity Score Matched Cohort Study. World J Surg 2017;41:516-24. [Crossref] [PubMed]
- Lee KF, Cheung YS, Chong CC, et al. Laparoscopic and robotic hepatectomy: experience from a single centre. ANZ J Surg 2016;86:122-6. [Crossref] [PubMed]
- Montalti R, Scuderi V, Patriti A, et al. Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc 2016;30:1004-13. [Crossref] [PubMed]
- Boggi U, Caniglia F, Vistoli F, et al. Laparoscopic robot-assisted resection of tumors located in posterosuperior liver segments. Updates Surg 2015;67:177-83. [Crossref] [PubMed]
- Govindarajan A, Arnaoutakis D, D'Angelica M, et al. Use of intraoperative ablation as an adjunct to surgical resection in the treatment of recurrent colorectal liver metastases. J Gastrointest Surg 2011;15:1168-72. [Crossref] [PubMed]
- Petrou A, Kontos M, Prassas E, et al. The three-surgeon technique for liver tissue dissection: towards real bloodless hepatectomy. J BUON 2012;17:304-9. [PubMed]
- Makki K, Chorasiya VK, Sood G, et al. Laparoscopy-assisted hepatectomy versus conventional (open) hepatectomy for living donors: when you know better, you do better. Liver Transpl 2014;20:1229-36. [Crossref] [PubMed]
- Dumitra S, Sun V, Ruel NH, et al. Wireless Real-Time Program Successfully Monitors Recovery after Major Abdominal Surgery. J Am Coll Surg 2016;223:e49. [Crossref]
- Warner SG, Jutric Z, Nisimova L, et al. Early recovery pathway for hepatectomy: data-driven liver resection care and recovery. Hepatobiliary Surg Nutr 2017;6:297-311. [Crossref] [PubMed]