Abstract
Background
Robot-assisted distal pancreatectomy (RDP) is increasingly used as an alternative to laparoscopic distal pancreatectomy (LDP) in patients with resectable pancreatic cancer but comparative multicenter studies confirming the safety and efficacy of RDP are lacking.
Methods
An international, multicenter, retrospective, cohort study, including consecutive patients undergoing RDP and LDP for resectable pancreatic cancer in 33 experienced centers from 11 countries (2010–2019). The primary outcome was R0-resection. Secondary outcomes included lymph node yield, major complications, conversion rate, and overall survival.
Results
In total, 542 patients after minimally invasive distal pancreatectomy were included: 103 RDP (19%) and 439 LDP (81%). The R0-resection rate was comparable (75.7% RDP vs. 69.3% LDP, p = 0.404). RDP was associated with longer operative time (290 vs. 240 min, p < 0.001), more vascular resections (7.6% vs. 2.7%, p = 0.030), lower conversion rate (4.9% vs. 17.3%, p = 0.001), more major complications (26.2% vs. 16.3%, p = 0.019), improved lymph node yield (18 vs. 16, p = 0.021), and longer hospital stay (10 vs. 8 days, p = 0.001). The 90-day mortality (1.9% vs. 0.7%, p = 0.268) and overall survival (median 28 vs. 31 months, p = 0.599) did not differ significantly between RDP and LDP, respectively.
Conclusions
In selected patients with resectable pancreatic cancer, RDP and LDP provide a comparable R0-resection rate and overall survival in experienced centers. Although the lymph node yield and conversion rate appeared favorable after RDP, LDP was associated with shorter operating time, less major complications, and shorter hospital stay. The specific benefits associated with each approach should be confirmed by multicenter, randomized trials.
Similar content being viewed by others
Minimally invasive distal pancreatectomy (MIDP) has become the preferred approach for most resectable lesions in the pancreatic body and tail.1 Two randomized trials and numerous retrospective studies have shown that MIDP, consisting of both laparoscopic distal pancreatectomy (LDP) and robot-assisted distal pancreatectomy (RDP), is associated with faster functional recovery compared with open distal pancreatectomy (ODP).2,3,4 Although MIDP is increasingly being used in patients with resectable pancreatic cancer,5,6 randomized, controlled trials confirming its safety and efficacy in this patient category are still lacking.
While MIDP is mainly performed through laparoscopy, the robot-assisted approach is gaining popularity.7 In general, for all indications, retrospective studies have suggested that RDP is associated with improved rates of spleen-preservation and conversion, and shorter hospital stay, compared with LDP.7,8,9,10 However, studies specifically in patients with pancreatic cancer are scarce and only consist of single-center or small, cohort studies.11,12
To date, an international comparison of RDP and LDP in a large cohort of patients with resectable pancreatic cancer in experienced centers is lacking. As the use of a robotic approach in distal pancreatectomy continues to increase, it is important to investigate its safety and efficacy in patients with pancreatic cancer. Therefore, the purpose of this study is to compare the surgical and oncological outcome of RDP versus LDP in patients with resectable pancreatic cancer in a large, international, multicenter cohort.
Methods
Study Population and Design
This retrospective study was performed among centers participating in the European Consortium on Minimal Invasive Pancreatic Surgery (E-MIPS) and one non-European center. Only centers who had performed at least 50 MIDP procedures for all indications were included. Consecutive patients undergoing MIDP for pancreatic ductal adenocarcinoma (PDAC) between January 1, 2010 and December 31, 2019 were screened for eligibility. Patients were excluded if they had a previous pancreatic resection or were considered as borderline or locally advanced pancreatic cancer at diagnosis according to the NCCN guidelines.13 Patients were categorized according to the surgical technique applied: RDP and LDP. Patients undergoing conversion were included according to the initial surgical approach. Primary outcome was the R0-resection rate. Secondary outcomes included lymph node yield, major complication rate, conversion rate, and overall survival.
At each participating center, a local coordinator was responsible for the communication with the central study coordinators (JC, SL). Participating centers provided anonymized data on a password secured database. The central study coordinators combined the data.
This study was performed according to the principles of the Declaration of Helsinki (64th Fortaleza Brazil, October 2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO) and STROBE guidelines on reporting on observational studies.14 Due to the retrospective design, the ethical board from Amsterdam UMC waived the need for informed consent.
Definitions
Pancreatic cancer was defined according to the WHO classification of pancreatic tumors as pancreatic ductal adenocarcinoma.15 Conversion was defined as any attempted minimally invasive resection requiring conversion to laparotomy for other reasons than trocar placement or specimen extraction.16 Conversions were classified as elective conversions if there were unexpected findings, such as progression of tumor into surrounding structures or difficulty achieving tumor exposure or dissection. Conversions were classified as emergency conversion if unexpected events occurred, for instance bleeding.10 Operation time was calculated from robotic docking until completion of the surgical procedure. Postoperative complications were classified according to the Clavien-Dindo classification.17 Major complications were defined as Clavien-Dindo grades ≥3a. The definitions of pancreatic surgery specific complications of the International Study Group of Pancreatic Surgery were used to define postoperative pancreatic fistula, delayed gastric emptying, and post-pancreatectomy hemorrhage.18,19,20 Only complications graded as B and C were noted. Data on surgical site infections or radiological interventions were not collected. Postoperative outcomes were recorded up to 90 days postoperatively. Resection margins, including transection and posterior margins, were by all centers similarly categorized into: R0 (distance margin to tumor ≥1 mm), R1 (distance margin to tumor <1 mm), and R2 (macroscopically positive margin) according to the Royal College of Pathologists definition.21 The tumors were classified according to the American Joint Committee on Cancer (AJCC) 8th edition staging system.22
Statistical Analysis
Data were analyzed by using IBM SPSS Statistics for Windows version 26.0 (IBM Corp., Orchard Road Armonk, NY). Analyses were performed according to the intention-to-treat principle. Normally distributed continuous data were presented as mean with standard deviations (SD) and were compared by using the two-tailed Student t-test. Nonnormally distributed continuous data were presented as median with interquartile range (IQR) and were compared using the Mann-Whitney U test or the Kruskal–Wallis test, as appropriate. Categorical data were presented as frequencies with percentages and were compared by using the chi-square or Fisher’s exact test, as appropriate. The overall survival and disease-free interval were calculated by using Kaplan-Meier estimates and reported until 36 months of follow-up. Overall survival was defined from the date of surgery until the date of death or loss of follow-up, and all patients who were alive at the last follow-up date were censored. Disease-free interval was defined from the date of surgery until the first recurrence or death. The log-rank (Mantel-Cox) test was used to compare survival probabilities. P < 0.05 was considered statistically significant. Additionally, multivariable logistic regression analyses were performed for the two main outcomes of the study: R0 resection and major complications to examine whether the surgical approach or other variables were significantly associated with both outcomes. Variables with p < 0.20 in univariable analysis or clinical relevance based on literature were considered for multivariable analysis. Multivariable logistic regression analysis was performed by using binary logistic regression with backward selection with a p < 0.10, presented as odds ratios (OR) with corresponding 95% confidence intervals (CI). P < 0.05 was considered statistically significant.
Results
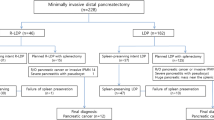
Overall, 542 patients after MIDP for resectable pancreatic cancer were included from 33 centers in 11 countries. Of the 542 patients, 103 patients (19%) underwent RDP and 439 patients (81%) LDP, without any differences in baseline characteristics between both groups (Table 1).
Intraoperative Outcomes
Intraoperative variables are presented in Table 2. RDP was associated with a longer operative time (290 vs. 240 minutes, p < 0.001) and more vascular resections (7.6% vs. 2.7%, p = 0.030). The rate of conversion to open surgery was significantly lower in the RDP group (4.9% vs. 17.3%, p = 0.001). No emergency conversions occurred during RDP compared with LDP (0% vs. 5.3%, p = 0.004). Both emergency conversions and elective conversions required longer operating time compared with procedures without conversion (295 and 280 vs. 234 minutes, p < 0.001).
Histopathological Outcomes
Histopathological variables are shown in Table 3. The R0-resection rate did not differ between RDP and LDP (75.7% vs. 69.3%, p = 0.404). The median lymph node yield was higher in RDP compared with LDP (18 vs. 16, p = 0.021), whereas no difference was observed in rate of positive lymph nodes between both groups (58.2% vs. 59.6%, p = 0.799).
Postoperative Outcome
Postoperative outcomes are presented in Table 4. Major complications occurred more frequently after RDP (26.2% vs. 16.3%, p = 0.019), whereas the rate of postoperative pancreatic fistula grade B/C (20.4% vs. 19.4%, p = 0.821), post-pancreatectomy hemorrhage grade B/C (2.9% vs. 3.0%, p = 0.953), and delayed gastric emptying (4.0% vs. 1.7%, p = 0.144) did not differ significantly between RDP and LDP, respectively. The median length of hospital stay was longer after RDP (10 vs. 8 days, p = 0.001). No differences were found in readmission and reoperation rates between both groups. The 30-day mortality (1.9% vs. 0.7%, p = 0.241) and 90-day mortality (1.9% vs. 0.7%, p = 0.268) did not differ between RDP and LDP, respectively.
The median follow-up time was 12 months (interquartile range [IQR] 6–12) for RDP and 18 months (IQR 10-30) for LDP. No differences were observed between both groups in overall survival (median RDP 28 vs. LDP 31 months, p = 0.602), as shown in Fig. 1A, and the disease-free interval (median RDP 21 vs. LDP 25 months, p = 0.366), as shown in Fig. 1B.
A Kaplan-Meier curve of overall survival in patients with resectable pancreatic cancer after robot-assisted distal pancreatectomy (RDP) and laparoscopic distal pancreatectomy (LDP). B Kaplan-Meier curve of disease-free interval of patients after robot-assisted distal pancreatectomy (RDP) and laparoscopic distal pancreatectomy (LDP)
Multivariable Regression Analyses
In the multivariable regression analysis of R0 resection, age ≥65 years (OR 1.86, 95% CI 1.11–3.13, p = 0.019), ASA classification of III–IV (OR 1.68, 95% CI 1.07–2.64, p = 0.024), intraoperative blood transfusion (OR 2.51, 95% CI 1.09–5.78, p = 0.031), and elective conversion (OR 2.39, 95% CI 1.25–4.58, p = 0.008) were associated risk factors for a R1 resection (Table 5).
Multivariable logistic regression analysis of potential variables associated with major complications revealed that only an ASA classification of III–IV was significantly associated with an increased risk of major complications (OR 1.81, 95% CI 1.09–3.00, p = 0.021) as shown in Table 6. RDP was not an associated risk factor when adjusted for other variables (OR 1.41, 95% CI 0.75–2.65, p = 0.29).
Discussion
This first international, multicenter, retrospective, cohort study comparing RDP and LDP in 542 patients with resectable pancreatic cancer from 33 centers in 11 countries found a comparable R0 resection margin and overall survival rate between RDP and LDP and a higher lymph node yield in RDP. Other notable differences were the lower conversion rate, higher rate of vascular resection, and higher rate of major complications in RDP, and a shorter operative time and shorter hospital stay in LDP. In multivariable analysis, RDP was not associated with major complications.
In recent years, MIDP has rapidly become the standard approach for symptomatic benign and low-grade malignant lesions requiring distal pancreatectomy.1 However, the oncological safety and efficacy of MIDP in patients with pancreatic cancer remains controversial and studies comparing RDP and LDP in patients with resectable pancreatic cancer are still scarce. First, the pan-European propensity score-matched DIPLOMA cohort study suggested that MIDP is associated with better short-term outcomes, i.e., less intraoperative blood loss and shorter hospital stay with a higher R0-resection rate, a higher lymph node yield, and comparable overall survival compared to ODP.5 Following on this, the same group recently completed the European, randomized, DIPLOMA-1 trial comparing MIDP and ODP in patients with resectable pancreatic cancer, and these results are expected soon.23 Recently, the first systematic review and meta-analysis comparing RDP with LDP in patients with pancreatic cancer included 6 retrospective studies, of which 5 single-center and 1 multicenter study, comprising a total of 572 patients (152 RDP, 420 LDP).24 The current study by itself included almost the same number of patients: 542 patients of 33 centers. The systematic review reported a higher R0 resection rate after RDP compared with LDP, without differences in operative time, tumor size, and lymph node yield. Only two studies, with in total 158 patients, reported on overall survival and found no differences between RPD and LDP.
The lower conversion rate in RDP as seen in the current study is in agreement with prior literature.6,8,25,26,27 This could be attributed to the technical capacity of the robotic platform, allowing for earlier and easier control of, for example, intraoperative bleeding, which may eventually be a reason for conversion. Furthermore, one-third of all conversions during LDP were emergency conversions against no emergency conversions during RDP. A previous study revealed that emergency conversions during MIDP are associated with increased overall morbidity and worse oncological outcome.10 A reduced conversion rate in RDP could be advantageous in this regard and therefore should be taken into consideration in the choice for the surgical approach of a distal pancreatectomy in patients with pancreatic cancer.
Remarkably, although the rate of major complications was 10% higher in the RDP group, the rates of postoperative pancreatic fistula, post-pancreatectomy hemorrhage, and delayed gastric emptying grade B/C were comparable between RDP and LDP. In multivariable, regression analysis, only an ASA III/IV classification was associated with major complications, a finding that has been described in previous literature.28,29 Also, there were proportionally more vascular resections performed in the RDP group (7.6% vs. 2.7%, p = 0.030). Although a correlation could not be proven, literature does suggest an association between vascular resections and major complications.30 On the other hand, the higher complication rate could be due to surgeons performing RDP during the first phase of their learning curve. Previous studies have proven that adoption of minimally invasive pancreatic surgery during the learning curve may cause increased morbidity rates.31,32 Unfortunately, this could not be verified in the present study, because no data were available on individual surgeons’ volume.
Regarding the oncological outcomes, a comparable R0-resection rate and higher lymph node yield was found after RDP compared with LDP. These results contradict the most recent systematic review, which reported a comparable lymph node yield and a higher R0 resection in RDP.24 However, the obtained difference should be interpreted with caution, given that it could possibly be influenced by differences in pathological examination protocols between centers rather than the quality of lymphadenectomy. For example, Sahakyan et al. demonstrated an increase in lymph node yield from 7 to 18 by standardizing the pathology examination without changing the surgical technique.33 In addition, the clinical relevance of the difference of only two lymph nodes could be questioned here, as no difference in positive lymph nodes or survival were observed between both groups. The comparable overall survival rates and disease-free intervals between RDP and LDP align with the results of a prior study that investigated the long-term outcomes between RDP and LDP in patients with pancreatic cancer in the National Cancer Database.6 These results indicate that the choice of approach does not impact patients’ survival.
The results of this study should be interpreted in light of several limitations. First, the retrospective design may have impacted the results as selection bias might be present and some important data were not available, such as on resection of Gerota’s fascia. Resection of Gerota’s fascia during distal pancreatectomy may improve oncological outcomes and therefore can have distorted the current comparison of both techniques.34 Second, no data on type of (neo)adjuvant treatment was available, although the use of neoadjuvant and adjuvant treatment in patients undergoing RPD and LDP was similar. FOLFIRINOX as adjuvant treatment has recently been associated with better overall survival in patients with resectable pancreatic ductal adenocarcinoma,35 so the obtained survival rates might be rather a reflection of this than the surgical technique. Third, the large number of centers participating in the study might have introduced heterogeneity. Although all participating centers had at least performed 50 MIDP procedures, their surgical technique as well as their experience on treating pancreatic cancer might differ. This also applies to the length of hospital stay, as outcomes may have varied due to different hospital discharge policies. Propensity score matching was considered for the current study, but eventually not performed due to comparable RDP and LDP groups and the potential loss of statistical power of matching. Fourth, no data on operative costs were collected. This is relevant given the high costs of the robotic system and also should be a topic in future prospective studies. A main strength of this study is the large sample size with a large number of centers reflecting current practice in 33 experienced centers from 11 countries.
Conclusions
This international cohort study, which compared RDP with LDP in patients with resectable pancreatic cancer in experienced centers, is the largest, retrospective cohort to date. It suggests that RDP is as oncologically safe as LDP by showing comparable R0-resection and survival rates with a higher lymph node yield. Because prospective studies comparing RDP with LDP are still lacking, future randomized studies, which could have a noninferiority design, are needed to prevent selection bias and identify those patients who will benefit from the potential advantages of a robot-assisted procedure.
References
Asbun HJ, Moekotte AL, Vissers FL, et al. The Miami International Evidence-based Guidelines on minimally invasive pancreas resection. Ann Surg. 2020;271(1):1–14.
de Rooij T, van Hilst J, van Santvoort H, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019;269(1):2–9.
Bjornsson B, Larsson AL, Hjalmarsson C, Gasslander T, Sandstrom P. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg. 2020;107(10):1281–8.
Abu Hilal M, Takhar AS. Laparoscopic left pancreatectomy: current concepts. Pancreatology. 2013;13(4):443–8.
van Hilst J, de Rooij T, Klompmaker S, et al. Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a pan-European propensity score matched study. Ann Surg. 2019;269(1):10–7.
Raoof M, Nota C, Melstrom LG, et al. Oncologic outcomes after robot-assisted versus laparoscopic distal pancreatectomy: analysis of the National Cancer Database. J Surg Oncol. 2018;118(4):651–6.
Zhou JY, Xin C, Mou YP, et al. Robotic versus laparoscopic distal pancreatectomy: a meta-analysis of short-term outcomes. PLoS One. 2016;11(3):e0151189.
Lof S, van der Heijde N, Abuawwad M, et al. Robotic versus laparoscopic distal pancreatectomy: multicentre analysis. Br J Surg. 2021;108(2):188–95.
Lyman WB, Passeri M, Sastry A, et al. Robotic-assisted versus laparoscopic left pancreatectomy at a high-volume, minimally invasive center. Surg Endosc. 2019;33(9):2991–3000.
Lof S, Korrel M, van Hilst J, et al. Outcomes of elective and emergency conversion in minimally invasive distal pancreatectomy for pancreatic ductal adenocarcinoma: an international multicenter propensity score-matched study. Ann Surg. 2021;274(6):e1001–7.
Qu L, Zhiming Z, Xianglong T, et al. Short- and mid-term outcomes of robotic versus laparoscopic distal pancreatosplenectomy for pancreatic ductal adenocarcinoma: a retrospective propensity score-matched study. Int J Surg. 2018;55:81–8.
Chopra A, Nassour I, Zureikat A, Paniccia A. Perioperative and oncologic outcomes of open, laparoscopic, and robotic distal pancreatectomy for pancreatic adenocarcinoma. Updates Surg. 2021;73(3):947–53.
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(8):1028–61.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9.
Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8.
Lof S, Vissers FL, Klompmaker S, et al. Risk of conversion to open surgery during robotic and laparoscopic pancreatoduodenectomy and effect on outcomes: international propensity score-matched comparison study. Br J Surg. 2021;108(1):80–7.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–91.
Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761–8.
Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–5.
Campbell FFA, Verbeke C. Dataset for the histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct 2010(261035):1–27.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (eds). AJCC Cancer Staging Manual. Vol 8: Springer International Publishing; 2017.
van Hilst J, Korrel M, Lof S, et al. Minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma (DIPLOMA): study protocol for a randomized controlled trial. Trials. 2021;22(1):608.
Feng Q, Jiang C, Feng X, et al. Robotic versus laparoscopic distal pancreatectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Front Oncol. 2021;11:752236.
Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257(1):128–32.
Liu R, Liu Q, Zhao ZM, Tan XL, Gao YX, Zhao GD. Robotic versus laparoscopic distal pancreatectomy: a propensity score-matched study. J Surg Oncol. 2017;116(4):461–9.
Xu SB, Jia CK, Wang JR, Zhang RC, Mou YP. Do patients benefit more from robot assisted approach than conventional laparoscopic distal pancreatectomy? A meta-analysis of perioperative and economic outcomes. J Formos Med Assoc. 2019;118(1 Pt 2):268–78.
Wang HB, Xiong GB, Zhu F, et al. Clavien-Dindo classification and influencing factors analysis of complications after laparoscopic pancreaticoduodenectomy. Zhonghua Wai Ke Za Zhi. 2018;56(11):828–32.
Sahakyan MA, Tholfsen T, Kleive D, et al. Laparoscopic distal pancreatectomy in patients with poor physical status. HPB (Oxford). 2021;23(6):877–81.
Feo CF, Deiana G, Ninniri C, et al. Vascular resection for locally advanced pancreatic ductal adenocarcinoma: analysis of long-term outcomes from a single-centre series. World J Surg Oncol. 2021;19(1):126.
Adam MA, Choudhury K, Dinan MA, et al. Minimally invasive versus open pancreaticoduodenectomy for cancer: practice patterns and short-term outcomes among 7061 patients. Ann Surg. 2015;262(2):372–7.
Sharpe SM, Talamonti MS, Wang CE, et al. Early national experience with laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma: a comparison of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg. 2015;221(1):175–84.
Sahakyan MA, Haugvik SP, Rosok BI, et al. Can standardized pathology examination increase the lymph node yield following laparoscopic distal pancreatectomy for ductal adenocarcinoma? HPB (Oxford). 2018;20(2):175–81.
Korrel M, Lof S, van Hilst J, et al. Predictors for survival in an international cohort of patients undergoing distal pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2021;28(2):1079–87.
Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–406.
Acknowledgment
For the European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS) Collaborators: Beatrice Aussilhou, MD, PhD12, Sivesh K. Kamarajah, MD, PhD31, Stijn van Laarhoven, MD, PhD20, Thomas Malinka, MD, PhD30, Ravi Marudanayagam, MD, PhD29, Claudio Ricci, MD, PhD9, Patricia Sánchez-Velázquez, MD, PhD7.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Disclosures
Prof. Marc Besselink and Prof. Mohammad Abu Hilal received a grant from Medtronic for investigator-initiated DIPLOMA randomized trial minimally invasive vs open distal pancreatectomy, a grant from Intuitive for investigator-initiated DIPLOMA-2 randomized trial minimally invasive pancreatoduodenectomy, as well as a grant from Ethicon for investigator-initiated for PANDORINA randomized trial drain placement distal pancreatectomy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jeffrey W. Chen and Tess M. E. van Ramshorst share first authorship.
Mohammad Abu Hilal and Marc G. Besselink share senior authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, J.W., van Ramshorst, T.M.E., Lof, S. et al. Robot-Assisted Versus Laparoscopic Distal Pancreatectomy in Patients with Resectable Pancreatic Cancer: An International, Retrospective, Cohort Study. Ann Surg Oncol 30, 3023–3032 (2023). https://doi.org/10.1245/s10434-022-13054-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-13054-2