Abstract
Purpose
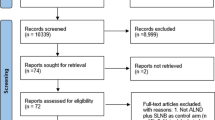
Several randomized controlled trials (RCTs) have investigated observation or axillary radiotherapy (ART) in place of completion axillary lymph node dissection (cALND) for management of positive sentinel nodes (SNs) in clinically node-negative women with breast cancer. The optimal treatment strategy for this population is not known.
Methods
MEDLINE, Embase, and EBM Reviews—NHS Economic Evaluation Database were searched from inception until July 2019. A systematic review and narrative summary was performed of RCTs comparing observation or ART versus cALND in clinically node-negative female breast cancer patients with positive SNs. The Cochrane risk of bias tool for RCTs was used to assess risk of bias. Outcomes of interest included overall survival (OS), disease-free survival (DFS), axillary recurrence, and axillary surgery-related morbidity.
Results
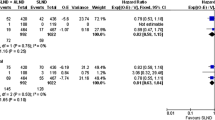
Three trials compared observation with cALND, and two trials compared ART with cALND. No studies blinded participants or personnel, and there was heterogeneity in inclusion criteria, study design, and follow-up. Neither observation nor ART resulted in statistically inferior 5- or 8-year OS or DFS compared with cALND. There was also no statistically significant increase in axillary recurrences associated with either approach. Four trials reported morbidity outcomes, and all showed cALND was associated with significantly more lymphedema, paresthesia, and shoulder dysfunction compared with observation or ART.
Conclusions
Women with clinically node-negative breast cancer and positive SNs can safely be managed without cALND.

Similar content being viewed by others
References
Klein S. Evaluation of palpable breast masses. Am Fam Physician. 2005;71(9):1731–1738.
Gradishar WJ, Anderson BO, Abraham J. NCCN Guidelines. Breast Cancer. 2019:215:9–11.
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(5):561–564. https://doi.org/10.1200/jco.2016.71.0947
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–398; (discussion 398–401).
Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(30):7703–7720. https://doi.org/10.1200/jco.2005.08.001
Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609. https://doi.org/10.1093/jnci/djj158
Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. https://doi.org/10.1016/s1470-2045(10)70207-2
Fisher ER, Redmond C, Fisher B. A perspective concerning the relation of duration of symptoms to treatment failure in patients with breast cancer. Cancer. 1977;40(6):3160–3167.
Fisher B, Jeong J-H, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567–575.
Black DM, Mittendorf EA. Landmark trials affecting the surgical management of invasive breast cancer. Surg Clin North Am. 2013;93(2):501–518. https://doi.org/10.1016/j.suc.2012.12.007
Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. https://doi.org/10.1001/jama.2011.90
Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. https://doi.org/10.1016/s1470-2045(14)70460-7
Sávolt Á, Péley G, Polgár C, et al. Eight-year follow up result of the OTOASOR trial: The optimal treatment of the axilla—surgery or radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2017;43(4):672–679. https://doi.org/10.1016/j.ejso.2016.12.011
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62(9):944–952. https://doi.org/10.1016/j.jclinepi.2008.10.012
Covidence—Better systematic review management. https://www.covidence.org/home. Accessed 14 March 2019.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Treadwell J, Uhl S, Tipton K, et al. Assessing Equivalence and Noninferiority. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. http://www.ncbi.nlm.nih.gov/books/NBK98979/. Accessed 30 July 2019.
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834.
7.7.7.2 Standard errors from confidence intervals and P values: difference measures. https://handbook-5-1.cochrane.org/chapter_7/7_7_7_2_obtaining_standard_errors_from_confidence_intervals_and.htm. Accessed 28 June 2019.
Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–432 https://doi.org/10.1097/sla.0b013e3181f08f32. (discussion 432–433)
Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264(3):413–420. https://doi.org/10.1097/sla.0000000000001863
Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. https://doi.org/10.1001/jama.2017.11470
Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305. https://doi.org/10.1016/s1470-2045(13)70035-4
Galimberti V, Cole BF, Viale G, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018. https://doi.org/10.1016/s1470-2045(18)30380-2
Solá M, Alberro JA, Fraile M, et al. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol. 2013;20(1):120–127. https://doi.org/10.1245/s10434-012-2569-y
Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group trial Z0011. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(24):3657–3663. https://doi.org/10.1200/jco.2006.07.4062
Jagsi R, Chadha M, Moni J, et al. Radiation field design in the ACOSOG Z0011 (Alliance) Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(32):3600–3606. https://doi.org/10.1200/jco.2014.56.5838
Torgerson DJ, Roland M. What is Zelen’s design? BMJ. 1998;316(7131):606. https://doi.org/10.1136/bmj.316.7131.606
van der Velden JM, Verkooijen HM, Young-Afat DA, et al. The cohort multiple randomized controlled trial design: a valid and efficient alternative to pragmatic trials? Int J Epidemiol. 2017;46(1):96–102. https://doi.org/10.1093/ije/dyw050
Cochrane Handbook for Systematic Reviews of Interventions. http://handbook-5-1.cochrane.org/. Accessed 28 Jan 2019.
Nardone L, Palazzoni G, D’Angelo E, et al. Impact of dose and volume on lymphedema. Rays. 2005;30(2):149–155.
Bentzen SM, Dische S. Morbidity related to axillary irradiation in the treatment of breast cancer. Acta Oncol Stockh Swed. 2000;39(3):337–347.
Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307–316.
Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–327. https://doi.org/10.1056/nejmoa1415369
Hennequin C, Bossard N, Servagi-Vernat S, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2013;86(5):860–866. https://doi.org/10.1016/j.ijrobp.2013.03.021
Budach W, Bölke E, Kammers K, Gerber PA, Nestle-Krämling C, Matuschek C. Adjuvant radiation therapy of regional lymph nodes in breast cancer - a meta-analysis of randomized trials- an update. Radiat Oncol Lond Engl. 2015;10:258. https://doi.org/10.1186/s13014-015-0568-4
Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03488693. Accessed 26 Dec 2019.
Ozcan LC, Giuliano AE. Is axillary lymph node dissection necessary after a positive sentinel lymph node biopsy? Adv Surg. 2017;51(1):165–178. https://doi.org/10.1016/j.yasu.2017.03.013
Glechner A, Wöckel A, Gartlehner G, et al. Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: a systematic review and meta-analysis. Eur J Cancer Oxf Engl 1990. 2013;49(4):812–825. https://doi.org/10.1016/j.ejca.2012.09.010
Huang T-W, Kuo KN, Chen K-H, et al. Recommendation for axillary lymph node dissection in women with early breast cancer and sentinel node metastasis: A systematic review and meta-analysis of randomized controlled trials using the GRADE system. Int J Surg Lond Engl. 2016;34:73–80. https://doi.org/10.1016/j.ijsu.2016.08.022
Schmidt-Hansen M, Bromham N, Hasler E, Reed MW. Axillary surgery in women with sentinel node-positive operable breast cancer: a systematic review with meta-analyses. SpringerPlus. 2016;5:85. https://doi.org/10.1186/s40064-016-1712-9
Zhao M, Liu W-G, Zhang L, et al. Can axillary radiotherapy replace axillary dissection for patients with positive sentinel nodes? A systematic review and meta-analysis. Chronic Dis Transl Med. 2017;3(1):41–50. https://doi.org/10.1016/j.cdtm.2017.01.005
de Boniface J, Frisell J, Andersson Y, et al. Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer. 2017;17(1):379. https://doi.org/10.1186/s12885-017-3361-y
Goyal A, Dodwell D. POSNOC: a randomised trial looking at axillary treatment in women with one or two sentinel nodes with macrometastases. Clin Oncol R Coll Radiol G B. 2015;27(12):692–695. https://doi.org/10.1016/j.clon.2015.07.005
Protocol 14PRT/0519. https://www.thelancet.com/doi/story/10.1016/html.2014.12.08.1563. Accessed January 28, 2019.
Houvenaeghel G, Cohen M, Raro P, et al. Overview of the pathological results and treatment characteristics in the first 1000 patients randomized in the SERC trial: axillary dissection versus no axillary dissection in patients with involved sentinel node. BMC Cancer. 2018;18(1):1153. https://doi.org/10.1186/s12885-018-5053-7
Houvenaeghel G, Resbeut M, Boher J-M. [Sentinel node invasion: is it necessary to perform axillary lymph node dissection? Randomized trial SERC]. Bull Cancer (Paris). 2014;101(4):358–363. https://doi.org/10.1684/bdc.2014.1916
Tinterri C, Canavese G, Bruzzi P, Dozin B. SINODAR ONE, an ongoing randomized clinical trial to assess the role of axillary surgery in breast cancer patients with one or two macrometastatic sentinel nodes. Breast Edinb Scotl. 2016;30:197–200. https://doi.org/10.1016/j.breast.2016.06.016
Acuna SA, Chesney TR, Amarasekera ST, Baxter NN. Defining non-inferiority margins for quality of surgical resection for rectal cancer: a Delphi consensus study. Ann Surg Oncol. 2018;25(11):3171–3178. https://doi.org/10.1245/s10434-018-6639-7
Acuna SA, Chesney TR, Ramjist JK, Shah PS, Kennedy ED, Baxter NN. Laparoscopic versus open resection for rectal cancer: a noninferiority meta-analysis of quality of surgical resection outcomes. Ann Surg. 2019;269(5):849–855. https://doi.org/10.1097/sla.0000000000003072
Acknowledgements
We thank Teruko Kishibe for her assistance with updating our search strategy.
Funding
None to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Final search strategy for EBM Reviews—NHS Economic Evaluation Database. The electronic databases MEDLINE, Embase, and EBM Reviews—NHS Economic Evaluation Database were searched from inception until July 2017, and a second updated search was performed from the previous search date until July 2019.

Appendix 2
10-Year overall survival, disease-free survival, and regional recurrence outcomes for two trials22,23,25
Characteristic | ACSOG Z0011 | IBCSG 23-01 | ||
|---|---|---|---|---|
cALND | Observation | cALND | Observation | |
Study design | Noninferiority | Noninferiority | ||
Length of follow-up, median (IQR), years | 9.3 (6.9–10.3) | 9.7 (7.8–12.7) | ||
OS, % (95% CI) | 83.6 (79.1–87.1) | 86.3 (82.2–89.5) | 88.2 (84.8–91.6) | 90.8 (87.9–93.8) |
HR (95% CI) | 0.85 (0.00–1.16)1 | 0.78 (0.53–1.14) | ||
DFS, % (95% CI) | 78.2 (73.5–82.2) | 80.2 (75.6–84.1) | 74.9 (70.5–79.3) | 76.8 (72.5–81.0) |
HR (95% CI) | 0.85 (0.62–1.17) | 0.85 (0.65–1.11) | ||
Axillary recurrence, % (95% CI) | 0.5 | 1.5 | 0.6 | 1.9 |
Chi squared p value | 0.276 | 0.083 | ||
HR (95% CI) | – | – | ||
Rights and permissions
About this article
Cite this article
Castelo, M., Hu, S.Y., Dossa, F. et al. Comparing Observation, Axillary Radiotherapy, and Completion Axillary Lymph Node Dissection for Management of Axilla in Breast Cancer in Patients with Positive Sentinel Nodes: A Systematic Review. Ann Surg Oncol 27, 2664–2676 (2020). https://doi.org/10.1245/s10434-020-08225-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08225-y