Abstract
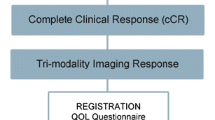
With current advances in neoadjuvant systemic therapy (NST) and improved breast imaging, the potential of nonoperative therapy for invasive breast cancer has emerged as a viable option when utilizing meticulous image-guided percutaneous biopsy to document pathologic complete response. Feasibility clinical trials utilizing this approach are being performed by teams of investigators from single and multicenter/cooperative groups around the world. Imaging alone after NST lacks sufficient sensitivity and specificity in predicting pCR and therefore cannot be utilized for clinical selection of patients for omission of surgery. Imaging with adequate sampling after NST of the residual lesions (or around the remaining clip if a complete radiologic response occurs) appears to be essential in selecting patients with pCR to lower the false-negative rates based on initial reported feasibility studies to identify pCR without surgery that range from 5 to 49%. In this manuscript, recently completed, ongoing, and planned clinical feasibility trials and a new omission of surgery trial are described. Drastic rethinking of all diagnostic and therapeutic management strategies that are ordinarily utilized for patients who receive standard breast cancer surgery is required. A roadmap of essential questions and issues that will have to be resolved as the field of nonoperative breast cancer management advances is described in detail.

Similar content being viewed by others
References
Rea D, Tomlins A, Francis A. Time to stop operating on breast cancer patients with pathological complete response? Eur J Surg Oncol. 2013;39(9):924–30. doi:10.1016/j.ejso.2013.1006.1005.
van la Parra RF, Kuerer HM. Selective elimination of breast cancer surgery in exceptional responders: historical perspective and current trials. Breast Cancer Res. 2016;18(1):28.
De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119(10):1776–83.
Heil J, Kummel S, Schaefgen B, et al. Diagnosis of pathological complete response to neoadjuvant chemotherapy in breast cancer by minimal invasive biopsy techniques. Br J Cancer. 2015;113(11):1565–70.
Heil J, Schaefgen B, Sinn P, et al. Can a pathological complete response of breast cancer after neoadjuvant chemotherapy be diagnosed by minimal invasive biopsy? Eur J Cancer. 2016;69:142–50.
Kuerer HM, Yang WT, Krishnamurthy S. Comment on “Diagnosis of pathological complete response to neoadjuvant chemotherapy in breast cancer by minimal invasive biopsy techniques.” Br J Cancer. 2016;114(10):e3.
Peintinger F, Kuerer HM, Anderson K, et al. Accuracy of the combination of mammography and sonography in predicting tumor response in breast cancer patients after neoadjuvant chemotherapy. Ann Surg Oncol. 2006;13(11):1443–9.
Schaefgen B, Mati M, Sinn HP, et al. Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol. 2015;23:789–5.
Marinovich ML, Houssami N, Macaskill P, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105(5):321–33.
Heil J. Diagnosis of pathological complete response by vacuum-assisted biopsy after neoadjuvant chemotherapy in breast cancer (RESPONDER). https://clinicaltrials.gov/ct2/show/NCT02948764. 2017.
Vrancken Peeters MJ. Towards omitting breast cancer surgery in patients without residual tumor after upfront chemotherapy. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6120. 2017.
Straver ME, Loo CE, Alderliesten T, Rutgers EJ, Vrancken Peeters MT. Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br J Surg. 2010;97(8):1226–31.
Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378–82.
Koolen BB, Donker M, Straver ME, et al. Combined PET–CT and axillary lymph node marking with radioactive iodine seeds (MARI procedure) for tailored axillary treatment in node-positive breast cancer after neoadjuvant therapy. Br J Surg. 2017. doi:10.1002/bjs.10555.
Frances A, Herring K, Molyneux S, et al. NOSTRA PRELIM: a non-randomized pilot study designed to assess the ability of image guided core biopsies to detect residual disease in patients with early breast cancer who have received neoadjuvant chemotherapy to inform the design of a planned trial. Cancer Res. 2017 (Proceedings of the 2016 San Antonio Breast Cancer Symposium). 2017;77(74 Suppl):16–14.
Kuerer HM, Rauch GM, Krishnamurthy S, et al. A clinical feasibility trial for identification of exceptional responders in whom breast cancer surgery can be eliminated following neoadjuvant systemic therapy. Ann Surg. 2017. doi:10.1097/SLA.0000000000002313.
Tadros AB, Krishnamurthy S, Yang WT, et al. Identification of patients with documented breast pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017. doi:10.1001/jamasurg.2017.0562.
Kuerer H. Eliminating breast cancer surgery in exceptional responders with neoadjuvant systemic therapy [ClinicalTrials.gov web site]. https://clinicaltrials.gov/ct2/show/NCT02945579. 2017.
Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8.
Teshome M, Kuerer HM. Breast conserving surgery and locoregional control after neoadjuvant chemotherapy. Eur J Surg Oncol. 2017.
Acknowledgement
Portions of this manuscript were presented at the 18th Annual American Society of Breast Surgeons Society general plenary session on April 29, 2017. This project was supported by the PH and Fay Etta Robinson Distinguished Professorship in Research endowment (HMK) and the National Institutes of Health (NIH) Cancer Center Support Grant (HMK, CA16672); Pink Ribbon NL (MJVP), Development Fund of the University of Birmingham for Surgical Research (DWR).
Disclosure
None of the authors has any commercial interest or conflict to disclose related to the present work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript is dedicated to the memory of Professor Adele Francis MBChB, PhD, FRCS a visionary leading breast surgeon and clinical investigator.
Rights and permissions
About this article
Cite this article
Kuerer, H.M., Vrancken Peeters, MJ.T.F.D., Rea, D.W. et al. Nonoperative Management for Invasive Breast Cancer After Neoadjuvant Systemic Therapy: Conceptual Basis and Fundamental International Feasibility Clinical Trials. Ann Surg Oncol 24, 2855–2862 (2017). https://doi.org/10.1245/s10434-017-5926-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5926-z