Abstract
Background
Reports show that FOLFIRINOX therapy for pancreatic ductal adenocarcinoma (PDAC) results in objective response rates two to threefold higher than those of other regimens. This study aimed to assess response and resection rates for locally unresectable (stage 3) patients initially treated with induction FOLFIRINOX.
Methods
The institutional cancer database was queried for patients treated with induction FOLFIRINOX therapy between 2010 and 2013. Patients were included in the study if they were treated at the authors’ institution for stage 3 PDAC (locally unresectable) that had been adjudicated at a weekly multidisciplinary tumor board.
Results
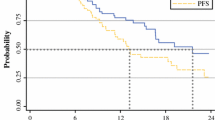
The study identified 101 patients. The median age was 64 years (range 37–81 years), and the median follow-up period was 12 months (range 3–37 months). The patients received a median of six cycles (range 1–20 cycles) of induction FOLFIRINOX. No grade 4 or 5 toxicity was recorded. At the initial restaging (median of 3 months after diagnosis), 23 patients (23 %) had developed distant metastases, 15 patients (15 %) had undergone resection, and 63 patients (63 %) had proceeded to chemoradiation. In the group of 63 patients who had proceeded to chemoradiation (median of 9 months after diagnosis), an additional 16 patients (16 %) had undergone resection, and 5 patients (5 %) had developed metastases. A partial radiographic response was observed in 29 % of all the patients, which was associated with ability to perform resection (p = 0.004). The median overall survival time was 11 months for the group that progressed with FOLFIRINOX and 26 months for the group that did not progress.
Conclusion
Nearly one third of the patients who had been initially identified as having stage 3 pancreatic carcinoma and had been treated with FOLFIRINOX responded radiographically and underwent tumor resection.



Similar content being viewed by others
References
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Marthey L, Sa-Cunha A, Blanc JF, et al. (2014) FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. doi:10.1245/s10434-014-3898-9.
Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543–8.
O’Reilly EM, Perelshteyn A, Jarnagin WR, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260:142–8.
Bickenbach KA, Gonen M, Tang LH, et al. Downstaging in pancreatic cancer: a matched analysis of patients resected following systemic treatment of initially locally unresectable disease. Ann Surg Oncol. 2012;19:1663–9.
Ammori JB, Colletti LM, Zalupski MM, et al. Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2003;7:766–72.
Cardenes HR, Moore AM, Johnson CS, et al. A phase II study of gemcitabine in combination with radiation therapy in patients with localized, unresectable, pancreatic cancer: a Hoosier Oncology Group study. Am J Clin Oncol. 2011;34:460–5.
Massucco P, Capussotti L, Magnino A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol. 2006;13:1201–8.
Small W Jr., Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008;26:942–7.
Aristu J, Canon R, Pardo F, et al. Surgical resection after preoperative chemoradiotherapy benefits selected patients with unresectable pancreatic cancer. Am J Clin Oncol. 2003;26:30–6.
Leone F, Gatti M, Massucco P, et al. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: a single institutional experience. Cancer. 2013;119:277–84.
Bajetta E, Di Bartolomeo M, Stani SC, et al. Chemoradiotherapy as preoperative treatment in locally advanced unresectable pancreatic cancer patients: results of a feasibility study. Int J Radiat Oncol Biol Phys. 1999;45:285–9.
Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002;6:763–9.
Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–7.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Version 1.2014. http://www.nccn.org//professionals/physician_gls/pdf/pancreatic.pdf. Accessed 10 Feb 2014.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
National Cancer Institute. Cancer Therapy Evaluation Program. Common Toxicity Criteria Manual, Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 20 Feb 2014.
Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–64.
DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–7; discussion 937–9.
Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–56.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. 2013;108:236–41.
Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12:199.
Acknowledgment
This study was funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748.
Disclosure
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sadot, E., Doussot, A., O’Reilly, E.M. et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol 22, 3512–3521 (2015). https://doi.org/10.1245/s10434-015-4647-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4647-4