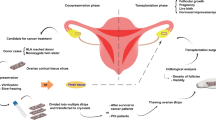
Abstract
Context
Ovarian cryopreservation followed by autotransplantation is still considered an experimental strategy for fertility preservation (FP) mainly because the success rates are unknown.
Objective
To determine cohort epidemiologic characteristics and success rates of autologous ovarian tissue transplantation (OTT) with previously cryopreserved tissue.
Materials and Methods
Literature review from 1999 to October 1, 2016. Additional cases were retrieved from meeting abstracts and own database. We selected studies that reported autologous OTT with previously banked tissue in humans. We did not include any cases involving fresh ovarian tissue transplantation or those performed to treat idiopathic premature ovarian failure/insufficiency. Both authors reviewed and selected studies for eligibility, which resulted in 59 full-text studies assessed for eligibility. Cases were extracted from original reports and reviews by the junior author, and the senior author reviewed and verified the extracted data.
Results
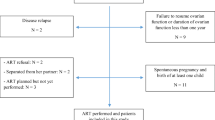
Nineteen reports were included for qualitative synthesis. In 10 studies, detailed data were available to determine clinical and live birth + ongoing (LB + OG) pregnancy as well as endocrine restoration rates. Three hundred nine OTTs were performed with cryopreserved tissue, resulting in the birth of 84 children and 8 OG pregnancies. The cumulative clinical and LB + OG rates were 57.5% and 37.7%, respectively, and the endocrine restoration rate was 63.9%.
Conclusion
Success rates with cryopre-served OTT have reached promising levels. Given these recent data, ovarian tissue cryopreservation should be considered as a viable option for FP.
Similar content being viewed by others
References
Parkes AS, Smith AU. Regeneration of rat ovarian tissue grafted after exposure to low temperatures. Proc R Soc Lond B Biol Sci. 1953;140(901):455–470.
Deanesly R. Immature rat ovaries grafted after freezing and thawing. J Endocrinol. 1954;11(2):197–200.
Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11(7):1487–1491.
Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13(5):1133–1138.
Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at e196 degrees C. Hum Reprod. 1994;9:597–603.
Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342(25):1919.
Oktay K, Oktem O. Fertility preservation medicine: a new field in the care of young cancer survivors. Pediatr Blood Cancer. 2009;53(2):267–273.
Oktay K, Buyuk E, Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363(9412):837–840.
Oktay K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: is there a germline stem cell connection? Hum Reprod. 2006;21(6):1345–1348.
Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010;93(3):762–768.
Oktay K, Bedoschi G, Pacheco FS, Turan V, Emirdar V. First pregnancies, live-birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94.e1–e9.
Azem F, Amit A, Vagman I, Mey Raz N, Ben-Yosef D, Lessing J. Successful pregnancy and birth following avascular micro ovarian tissue orthotopic transplantation in a patient with Hodgkin disease. Scientific Congress Supplement: oral and poster abstracts. Fertil Steril. 2012;98(3):S95–S96.
Donnez J, Dolmans MM, Pellicer A, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–1513.
Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–1170.
Dunlop CE, Brady BM, McLaughlin M, et al. Re-implantation of cryopreserved ovarian cortex resulting in restoration of ovarian function, natural conception and successful pregnancy after haematopoietic stem cell transplantation for Wilms tumour. J Assist Reprod Genet. 2016;33(12):1615–1620.
Fabbri R, Pasquinelli G, Magnani V, et al. Autotransplantation of cryopreserved ovarian tissue in oncological patients: recovery of ovarian function. Future Oncol. 2014;10(4):549–561.
Imbert R, Moffa F, Tsepelidis S, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29(9):1931–1940.
Demeestere I, Simon P, Dedeken L, et al. Live birth after auto-graft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30(9):2107–2109.
Jensen AK, Kristensen SG, Macklon KT, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod. 2015;30(12):2838–2845.
Kim SS, Lee WS, Chung MK, Lee HC, Lee HH, Hill D. Long-term ovarian function and fertility after heterotopic autotransplantation of cryobanked human ovarian tissue: 8-year experience in cancer patients. Fertil Steril. 2009;91(6):2349–2354.
Kiseleva M, Malinova I, Komarova E, Shvedova T, Chudakov K. The Russian experience of autotransplantation of vitrified ovarian tissue to a cancer patient. Gynecol Endocrinol. 2014;30(suppl 1): 30–31. doi:10.1007/s10815-016-0805-2.
Lorenzo F, Villamayor M, Viola J, Tiveron M, Young E. Second children born after autotransplantation of cryopreserved ovarian tissue in a young patient previously treated with chemotherapy for Askin’s disease. The successful of fertility preservation program. Fertil Steril. 2016;106(suppl 3):e29.
Meirow D, Ra’anani H, Shapira M, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106(2):467–474.
Póvoa A, Xavier P, Calejo L, et al. First transplantation of cryopreserved ovarian tissue in Portugal, stored for 10 years: an unexpected indication. Reprod Biomed Online. 2016;32(3):334–336.
Silber S, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reprod Biomed Online. 2015;30(6):643–650.
Stern K, Rozen G, Gook D, Agresta F, Braat D, Hale L. Are there factors which predict the success of ovarian tissue grafting in oncofertility patients? Abstract presented at: 32nd Annual Meeting of the European Society of Human Reproduction and Embryology; 3–6 July 2016; Helsinki, Finland.
Tanbo T, Greggains G, Storeng R, Busund B, Langebrekke A, Fedorcsak P. Autotransplantation of cryopreserved ovarian tissue after treatment for malignant disease—the first Norwegian results. Acta Obstetr Gynecol Scand. 2015;94(9):937–941.
Van der Ven H, Liebenthron J, Beckmann M, et al. FertiPROTEKT Network. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31(9):2031–2041.
Donnez J, Dolmans MM, Diaz C, Pellicer A. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104(5):1097–1098.
Callejo J, Salvador C, Gonzalez-Nunez S, et al. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res. 2013;6(1):33.
Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12(12):1437–1442.
Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil Steril. 2015;103(2):462–468.
Rodriguez-Wallberg KA, Karlstrom PO, Rezapour M, et al. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstetr Gynecol Scand. 2015;94(3):324–328.
Oktay K, Aydin BA, Karlikaya G. A technique for laparoscopic transplantation of frozen-banked ovarian tissue. Fertil Steril. 2001;75(6):1212–1216.
Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):18–21.
Silber SJ, DeRosa M, Pineda J, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Human Reprod. 2008;23(7):1531–1537.
Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410.
Donnez J, Silber S, Andersen CY, et al. Children born after auto-transplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–450.
Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93(7):2413.e15–e19.
Revelli A, Marchino G, Dolfin E, et al. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril. 2013;99(1):227–230.
Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286(12):1490–1493.
Stern CJ, Gook D, Hale LG, et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Hum Reprod. 2013;28(11):2996–2999.
Webber L, Davies M, Anderson R, et al. European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Human Reprod. 2016;31(5):926–937.
Oktay K, Buyuk E. Ovarian transplantation in humans: indications, techniques and the risk of reseeding cancer. Eur J Obstet Gynecol Reprod Biol. 2004;113(suppl 1):S45–S47. doi:10.1371/journal.pone.0019475.
Sonmezer M, Oktay K. Fertility preservation in female patients. Human Reprod Update. 2004;10(3):251–266.
Curtin JP, Barakat RR, Hoskins WJ. Ovarian disease in women with breast cancer. Obstet Gynecol. 1994;84(3):449–452.
Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol. 2015;33(22):2424–2429.
Stern CJ, Gook D, Hale LG, et al. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Human Reprod. 2014;29(8):1828.
Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology. 1999;140(1):462–471.
Bromer JG, Patrizio P. Fertility preservation: the rationale for cryopreservation of the whole ovary. Semin Reprod Med. 2009;27(6):465–471.
Bedaiwy MA, Hussein MR, Biscotti C, Falcone T. Cryopreservation of intact human ovary with its vascular pedicle. Human Reprod. 2006;21(12):3258–3269.
Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PloS One. 2011;6(4):e19475.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacheco, F., Oktay, K. Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis. Reprod. Sci. 24, 1111–1120 (2017). https://doi.org/10.1177/1933719117702251
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117702251