Article Text
Abstract
Objective To characterise published evidence regarding preclinical and clinical interventions to overcome mask shortages during epidemics and pandemics.
Design Systematic scoping review.
Settings All healthcare settings relevant to epidemics and pandemics.
Search strategy English peer-reviewed studies published from January 1995 to June 2020 were included. Literature was identified using four databases (Medline-OVID, EMBASE, CINAHL, Cochrane Library), forwards-and-backwards searching through Scopus and an extensive grey literature search. Assessment of study eligibility, data extraction and evidence appraisal were performed in duplicate by two independent reviewers.
Results Of the 11 220 database citations, a total of 47 articles were included. These studies encompassed six broad categories of conservation strategies: decontamination, reusability of disposable masks and/or extended wear, layering, reusable respirators, non-traditional replacements or modifications and stockpiled masks. Promising strategies for mask conservation in the context of pandemics and epidemics include use of stockpiled masks, extended wear of disposable masks and decontamination.
Conclusion There are promising strategies for overcoming face mask shortages during epidemics and pandemics. Further research specific to practical considerations is required before implementation during the COVID-19 pandemic.
- infection control
- public health
- risk management
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is the first scoping review of the literature that has evaluated the evidence behind overcoming mask shortages during pandemics and epidemics, which is increasingly relevant during the COVID-19 pandemic.
Strengths of design include the robust search strategy, thorough grey literature search, registration of protocol, multiple evidence appraisals and completion of all steps in duplicate with two reviewers.
Limitations include the limits of the evidence base and limitation to the English language.
Introduction
Face masks, including surgical masks and N95 respirators (table 1), are integral components of personal protective equipment (PPE) to protect healthcare workers (HCWs) from transmission of viral and bacterial pathogens.1 They are essential for the prevention of nosocomial infection of the current COVID-19 pandemic.2 The Centers for Disease Control and Prevention (CDC), WHO and expert bodies have highlighted the importance of appropriate PPE to prevent nosocomial infection of HCWs, as well as to limit the global spread of the virus.3–5 While there is controversy regarding whether community members should wear masks in public, there is a consensus that healthcare providers have greater risk of exposure and require protection.6 7 The consequences of limited or inappropriate use of PPE for healthcare providers has been demonstrated in previous epidemics and pandemics, including SARS, Ebolavirus and H1N1 influenza A.8–10
Types of face masks
Recently, WHO has called attention to shortages in face masks during the COVID-19 pandemic.11 The causes of these shortages are multifactorial, including increased demand for masks both by HCWs worldwide, and disruptions in the global supply chain through a large reduction in exports from China, a major producer of medical grade masks.12 Hoarding and misuse by lay people further compromises supply in times of mass panic.2 Given the currently high rate of infection of providers with COVID-19,13 14 maintaining an adequate supply for them is a matter of urgency.
Strategies for overcoming the limited supply of masks in this time of public health crisis are being prioritised by medical bodies. The CDC has released a document outlining potential organisational methods, reuse of disposable products, non-traditional mask sources and novel approaches for fabrication.15 The Journal of the American Medical Association (JAMA) recently issued a Call for Ideas for unconventional pitches related to increasing the PPE supply.16 While numerous editorials and news articles address this topic, we are unaware of a systematic search of the published research to date.17 18
The objective of this scoping review is to characterise the research outcomes for preclinical and clinical interventions for overcoming limited supply of masks during pandemics and epidemics. We hope to inform best practices for addressing the current and potential future shortage of PPE supply while still maintaining both patient and provider safety.
Methods
The scoping review was conducted according to the standards and guidelines established in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) with the associated extension for Scoping Reviews, in addition to the fourth edition of the Joanna Briggs Institute Reviewer’s Manual.19 20 We registered an iterative protocol through the Open Science Forum.21 22 Changes to the protocol were minimal, including one change to the search criteria to broaden the search by adding keyword searches.
Search strategy
We conducted a systematic literature search of Medline-OVID, EMBASE, CINAHL and Cochrane Library. Databases were examined from 1995 until the date of our literature searches (4 June 2020). The cut-off of 1995 was designated in order to balance relevance to newer mask models and infection control guidelines, while still including major epidemics such as SARS in 2003. A copy of the search strategy is provided in the online supplemental appendix 1.
Supplemental material
To ensure completeness, we also searched the references of our full-text articles, as well as the citing articles via Scopus. We also screened the references of identified relevant reviews.
Non-database sources were systematically searched to examine grey literature as well as to identify further peer-reviewed articles that may have been missed in the search. To identify relevant peer-reviewed articles, we hand-searched GoogleFoam,23 COVID-19 Expert,24 relevant guidelines,4 5 25–29 preprint databases28 29 and specialised evidence collections that were specific to the current COVID-19 pandemic.30–36 Sources of grey literature included DuckDuckGo,30 Google News,31 the JAMA Call to Ideas forum16 and LexisNexis.32 Details of the grey literature sources are listed in table 2. The sources of grey literature were selected by two frontline clinicians and senior authors (JMB, SMF) on the basis of relevance to the field.
Sources hand-searched for peer-reviewed literature
Articles were excluded if they did not report outcomes, were not specific to pandemics or epidemics, did not include English translations or were only relevant for a community setting. Details of the eligibility criteria are provided in box 1.
Eligibility criteria
Population:
Relevant to healthcare providers/hospital staff/medical institutions/long-term care homes/dental offices/paramedics and prehospital care workers/military medical services/refugee health workers or any medical institutions that use face masks for medical purposes. Face masks include surgical masks and non-powered respirators.
Intervention:
Any intervention with the purpose of conserving/rationing masks relevant to pandemics/epidemics; any intervention with the purpose of increasing the supply of masks through procurement from other sources relevant to pandemics/epidemics.
Comparator:
Not available (any identified from literature).
Outcomes:
Any outcome reported in the literature (can be qualitative or quantitative, may include patient outcomes/provider outcomes, may include increases to supply, may include other markers of clinical quality of performance).
Study selection
Each title/abstract identified from the database search underwent two rounds of screening by two independent reviewers. A total of four independent reviewers (AK, SK, TG, MY) participated in the screening process, with each reviewer reviewing half of the yield. A pilot test of the title/abstract screening was completed among the four reviewers for the first 200 search results to ensure sufficient inter-rater agreement. Afterwards, two reviewers (AK, SK) examined full-texts to assess for eligibility. Any disagreements between the two reviewers was resolved through discussion and consultation with the two senior authors (JMB, SMF).
Data extraction
To facilitate data extraction, a standardised form was developed and piloted on five studies. The data extraction template was modified in an iterative process until the research team was satisfied with its state. Two reviewers (AK, SK) piloted extraction for five studies with each other for the purpose of improving the extraction process.
Following the pilot, the full data extraction was completed by the four reviewers (AK, SK, TG, MY) working in parallel. Any disagreements in data extraction were resolved through discussion and consultation with the content experts (JMB, SMF). Summary and synthesis were completed descriptively.
Quality assessment and risk of bias
The quality rating of all studies was also graded in duplicate by two reviewers (AK, SK) using a rating scale adapted from the Oxford Centre for Evidence-based Medicine.37
The risk of bias of the included studies was then systematically assessed by at least two independent reviewers (AK, SK, JMB). Non-randomised trials were evaluated using the RoBANS tool, while randomised controlled trials (RCTs) were evaluated using the Cochrane risk of bias tool. To our knowledge, there is no widely accepted measure of quality for preclinical studies. As such, we adapted approaches previously reported in the literature to select five markers of quality for our included preclinical studies.38–42
Patient and public involvement
Patients and members of the public were not involved in the conduction of this scoping review.
However, this review was conducted under the supervision of two academic emergency physicians who serve on the frontlines during the COVID-19 pandemic. The relevance of the research question and outcome measures were thus informed by their priorities, experiences, l and preferences as HCWs.
Results
Search yield
Results of the study screening process are available in the PRISMA diagram in figure 1. Of the 11 220 imported titles and database citations, 5038 remained after duplicates were removed. After title and abstract screening, 71 were eligible for full-text evaluation. Of the 71 full-text articles, a total of 47 met inclusion criteria for this scoping review.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis diagram.
Article characteristics
Full details of the included articles are available in the online supplemental appendix 2.
Supplemental material
All 47 studies were full-text articles. Of the 47 studies, 27 were laboratory-based. The remainder were user acceptance studies (n=5) or clinical designs (n=15). Of the 15 clinical studies, 7 were RCTs and the remainder were non-randomised/observational (n=8).
The majority of studies were conducted in the USA (n=39), with the remainder located in Asia (n=4), South America (n=1), Africa (n=1) or a combination of countries (n=2).
There were 25 studies that were specific to N95 respirators, with the remainder evaluating cloth masks (n=2), surgical masks (n=2), reusable elastomeric respirators (n=6) or multiple types of masks (n=12).
Twenty studies reported no conflict of interest. One study43 noted that an author had a previous financial relationship with 3M.43 This same study reported receiving support from 3M for mask testing. Two other studies44 45 reported receiving support from industry partners.44 45 Of these, one stated the authors had no conflicts of interest, and one did not include any statement of potential conflicts of interest. The remaining 24 studies did not provide a disclosure statement.
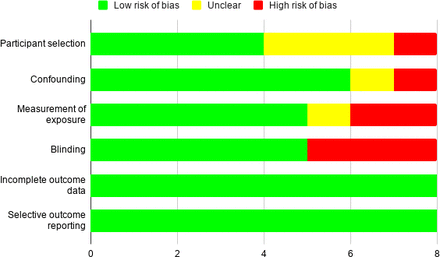
Details of the evidence grading and risk of bias assessment are available in the online supplemental appendix 2 as well as in figures 2–4.
Cochrane risk of bias tool. Seven randomised controlled trials were evaluated using the Cochrane risk of bias tool. The majority (n=6) were noted to be intermediate risk, with one study graded as low risk.
RoBANS risk of bias tool. Eight non-randomised studies were evaluated with the RoBANS tool. Seven studies were graded as low risk, with one study graded as intermediate risk to high risk.
Preclinical risk of bias grading. Twenty-seven preclinical studies were evaluated using markers previously described in literature (see ‘Methods’ section). All studies were ranked as medium to high risk for bias.
Strategies for overcoming limited supply
The research literature revealed numerous strategies evaluated for overcoming a limited supply of PPE during pandemics or epidemics. These strategies can be grouped into six main categories (table 3): decontamination of disposable masks, reuse and/or extended wear of disposable masks, layering of masks, introduction of reusable respirators, use of non-traditional replacements or modifications to masks, and use of stockpiled or expired masks.
Description of strategies
Summary of decontamination methods
Decontamination of disposable masks
Eighteen of the included studies evaluated decontamination methods of disposable masks in order to facilitate reuse. There were multiple methods of decontamination including: ultraviolet (UV) germicidal irradiation, pasteurisation, dry heat and chemical disinfectants (including ethylene oxide, ammonia, hydrogen peroxide, bleach, isopropyl alcohol, mixed disinfectants and commercially available cleaning wipes). A full summary of decontamination methods and assessment using the Health Canada criteria for mask decontamination is included in table 4.46
Studies of mask decontamination incorporated one or more of four outcome measures: (1) decontamination efficacy, (2) filtration performance after decontamination, (3) complications of decontamination, (4) user experience/acceptance of decontamination. Fifteen studies evaluated the efficacy of methods for decontamination of filtering facepiece respirators, including N95s and P100s. These were conducted in controlled laboratory settings, where primary outcomes included changes in viability of live pathogens and filtration performance on decontamination. Evaluated pathogens included strains of H1N1 (n=3), MS2 bacteriophage (n=4), Escherichia coli (n=1), Bacillus subtilis (n=1), Geobacillus stearothermophilus (n=1) and Staphylococcus aureus (n=1).47–51 All studies noted some degree of reduced virus viability with UV, chemical or heat-based decontamination methods. The most studied method of decontamination was UV radiation, with 13 studies evaluating either UVA or UVC radiation at varying doses and exposure times (details in table 5). While most studies found most decontamination methods to be effective, UVC radiation (15 W 254 nm bulbs for 15 min) was noted as the most effective method by Lore et al50 in comparison to microwave-generated steam or moist heat. In addition, decontamination using non-medical commercially available wipes and ethanol was notably ineffective.52 53 In the only available comparison of UVC and UVA, UVA was found ineffective compared with UVC.53
Summary of studies evaluating UVC decontamination
There were contrasting results regarding filtration performance and decontamination methods. Several studies found diminished filtration performance on decontamination with bleach, ethylene oxide, ethanol, autoclaves, rice cookers or microwave heat.52 54 55 Viscusi et al55 found that UV and hydrogen peroxide (liquid and vaporised) had the least effect on filter performance. However, Bergman et al56 found that, with the exception of hydrogen peroxide gas plasma which performed poorly, all treatment and control groups had comparable impact on filtration performance. Similarly, Fisher et al noted that microwave steam bags were 99.9% effective in MS2 decontamination while maintaining filtration efficiency.47
There were several complications associated with decontamination. For example, microwave irradiation using dry heat was noted to melt several filtration facepiece respirator (FFR) models.54 57 Decontamination using ethylene oxide created hazardous by-products that could be injurious to provider.58 Bleach would often impart a discernible odour on the FFR as well as corrode metal parts, such as the nose clip of masks.58 59 Physical degradation also occurred in a dose-dependent manner with UV treatment and after repeated hydrogen peroxide treatment.51 60 However, most studies did not formally assess mask fit after decontamination (table 4).
Two studies analysed the determinants related to provider uptake of decontamination.61 62 Nemeth et al61 evaluated user acceptance of FFR decontamination, noting that perceived safety of UV decontamination was higher in comparison to wearing an FFR for an extended period of time without decontamination.61 Viscusi et al62 reported that decontamination with UV, moist heat or microwave steam did not significantly change the user experience. Their clinical study found that FFR users are not likely to experience clinically meaningful reduction in fit, or an increase in odour, discomfort or difficulty in donning after decontamination. However, the authors noted that their results may have limited generalisability, as participants only wore the masks for 30 min when assessing comfort.
Reusability and extended wear of disposable masks
Ten studies evaluated outcomes related to the reusability and extended wear of disposable masks. All 10 studies evaluated N95 respirators, while 2 studies additionally evaluated surgical masks. Details of the studies are provided in table 6.
Summary of studies involving the reusability or extended wear of disposable masks
Three studies were laboratory-based.63–65 Coulliette et al63 noted that H1N1 viruses remained infectious for 6 days when deposited on the respirators under several conditions. Similarly, Fisher et al64 found that respirators have the potential to act as fomites, as MS2 bacteriophage were still detectable on the 10th day after deposition. Another study considered contamination with extended use, by quantifying the reaerosolisation of MS2 bacteriophage due to reverse airflow after simulated coughing. They found that <1% of viable virus was reaerosolised after a single cough.
Of the six clinical studies, two examined the performance of N95s after extended use in a healthcare setting. Duarte et al assessed the physical damage of N95 respirators over 1–30 days of consecutive use.66 A total of 668 respirators worn by 167 nursing assistants were evaluated. Past the fifth day of consecutive use, the respirators were visibly contaminated and folded. However, this was a subjective assessment of mask damage and was limited to visual characteristics. In contrast, Brady et al67 presented a more controlled clinical study that assessed pathogen transfer after reuse of N95s. Their results found that adequate doffing procedures had a greater impact in preventing contamination than whether a mask was reused. Specifically, MS2 bacteriophage contamination was lower with reuse and proper doffing in comparison to improper doffing.
Two studies analysed perceived discomfort and exertion of HCWs on extended wear of the masks. Radonovich et al68 noted that participants discontinued N95 use before 8 hours in 59% of sessions, citing intolerance. Similarly, Shenal et al69 noted that perceived discomfort increased over an 8-hour period, but exertion only marginally increased. In addition, two studies noted that fit testing scores of respirators dropped significantly with multiple wears. Specifically, fit factor consistently dropped after a maximum of five consecutive donnings and half of participants failed at least one fit test after repeated donning and doffing.70 71
Finally, Pillai et al72 conducted a survey of physician preferences regarding conservation strategies in N95 shortages. They noted that extended and reuse of disposable N95s was the most preferred conservation strategy, in comparison to use of reusable respirators.72
Layering of multiple masks
Five studies evaluated outcomes related to layering multiple masks, including layering the same mask type (n=1) versus overlay of one mask model over another (n=4). Details of the included studies are outlined in table 7.
Summary of studies involving the layering of multiple masks
Derrick et al73 evaluated combinations of one, two, three or five surgical masks overlayed on top of one another in a crossover study of six volunteers. They noted that while combining multiple surgical masks improved filtration, this was still well below that of N95 respirators.73
Three clinical studies evaluated user experience of surgical mask overlay over N95s.69 74 75 Shenal et al69 and Roberge et al75 found no statistically significant differences between overlay versus N95 respirator on its own. In contrast, Rebmann et al74 found that the overlay was perceived to be less comfortable and raised CO2 levels significantly, but without clinically relevant outcomes.74
Finally, a laboratory study found that the effect of a surgical mask overlay had variable effects depending on the model of N95.76 For cup models, this worsened respiratory gases, but for horizontal models it improved or did not change these values. The authors suggested that the differences would likely be imperceptible at low levels of exertion, however, no clinical correlates were evaluated.
Introduction of reusable respirators
Seven studies evaluated the use of reusable respirators as a method of conservation for disposable masks (table 8).
Summary of studies involving reusable respirators
Two laboratory-based studies evaluated the efficacy of decontamination of reusable respirators.77 78 Both studies reported that chemical disinfectant wipes (combined isopropyl alcohol plus quaternary ammonium wipes) were effective against influenza, but Subhash et al78 found that isopropyl alcohol alone was ineffective.
The remaining five studies analysed the logistics and feasibility of introducing reusable respirators. Bessesen et al79 noted that creation of standard operating procedures for disinfection significantly reduced the number of errors made by HCW, in comparison to following manufacturer instructions.79 In addition, Pompeii et al80 found that HCWs can be rapidly fit tested and trained to use the reusable elastomers in an outbreak simulation. Reusable elastomers did not require significantly different fit times in comparison to N95 fit testing.
Finally, three studies by Hines et al81–83 evaluated user preferences and driving factors behind reusable elastomer programmes via surveys, focus groups and interviews. Reasons for adoption included perception that elastomers are more protective and useful during N95 shortages. Concerns for adoption included lack of convenience, dissatisfaction with breathing when wearing the respirator and obstacles to access disinfection services. Other barriers to compliance and continued use were lack of availability, difficulties with storage, and difficulties changing filters.
Unconventional mask replacements or modifications
Three studies evaluated non-traditional reusable masks43–45 (table 9). Au et al45 tested a reusable plastic mask trimmed to the user’s face via an unblinded RCT. They noted that N95s were more effective in reducing airborne particles than the reusable masks. Two studies evaluated reusable cloth masks. MacIntyre et al43 conducted a multi-institute RCT in a low-resource setting, in which reusable cloth masks were provided to 569 HCWs. Five double-layer cotton masks were provided to each worker for the four consecutive weeks, to be washed with soap and water each day. The rate of wearer respiratory infection was significantly higher in the cloth mask arm versus the medical mask controls, with laboratory tests also noting higher penetration of particles through the cloth masks. Similarly, Rengasamy et al44 conducted a laboratory investigation in which cloth masks made from sweatshirts, T-shirts, towels, scarves and cotton were evaluated. They noted a wide variation in penetration across different fabrics, with higher penetration in cloth masks versus N95 controls.44
Summary of studies involving unconventional mask replacements or modifications
Another preclinical study evaluated the creation of a reusable virus deactivation system built into surgical masks. The investigators coated the middle of the three-layer masks (the polypropylene microfiber filter layer) with a solution of 29.03 wt by volume% of NaCl.84 They noted that salt-coated filters had higher filtration efficiency against influenza viruses, in comparison to bare filters. Mice who were protected against H1N1 by salt filters showed higher survival rate in comparison to mice who were unprotected. The authors additionally noted that the salt-coated filters were effective in a variety of storage conditions.
Stockpiled or expired masks
Four studies evaluated the performance of respirators after stockpiling or storage (table 10). All four studies had favourable results in quality testing of stockpiled masks.
Summary of studies involving the stockpiling or use of expired masks
Greenawald et al85 evaluated almost 4000 masks at 10 stockpile facilities in the USA with varying humidity and temperature parameters. All masks were tested beyond their listed expiration date, which ranged from over 5 to 10 years old. They found that 98% of tested N95s met performance standards for filtration performance, with only 2% of respirators having visual inspection concerns. Similarly, Viscusi et al57 determined that most models stored for up to 10 years in warehouses had adequate filtration performances.
Bergman et al86 found that the majority of respirator models in storage had adequate fit for subjects. However, Rottach et al87 found that strap strength across time of storage was model-dependent. While one model showed no clear difference with age, another manufacturer’s strap decreased in tensile strength over time.
Summary of grey literature
There were numerous diverse suggestions in the grey literature for potential conservation strategies. However, we found no included evaluations or outcomes, and no peer-reviewed studies that had not already been captured in our review. Examples of the conservation strategies are listed in table 11.
Results of non-peer-reviewed literature
Discussion
We included 47 studies in our systematic scoping review to characterise interventions related to overcoming limited supply of masks during pandemics and epidemics. These studies encompassed six broad categories of conservation strategies: decontamination, reusability of disposable masks and/or extended wear, layering, reusable respirators, non-traditional replacements or modifications and stockpiled masks.
Almost half of the included studies were laboratory-based or preclinical, while the remainder were user acceptance studies or clinical designs. A number of promising strategies were identified, including the use of reusable respirators, extended wear of N95s, use of masks stockpiled beyond manufacturer’s listed expiry date and decontamination. While numerous studies suggested that decontamination of masks is feasible, there were three potential caveats that require further study: (1) hazardous by-products, (2) physical degradation and (3) compromise of mask fit. Strategies that were found to be less effective included the use of cloth masks, layering multiple surgical masks or re-donning previously used masks that have not been sterilised. Barriers to mask conservation strategies included the time costs, necessary training and provider compliance. Strategies such as the creation of standardised operating procedures, physician education and user feedback were proposed to overcome these barriers.
However, the generalisability of these findings is limited. Minimum evidence requirements from regulatory agencies such as Health Canada include: demonstration that number of pathogens has been reduced, demonstration that respirator filter and fit performance are maintained, evidence that there is no residual chemical hazard and assurance of adequate labelling.46 The available literature does not meet these standards given the relative paucity of clinical studies. Many of the preclinical studies did not evaluate practical logistical barriers towards usage. For example, many studies cut N95 respirators into smaller coupons in order to test various decontamination techniques, precluding any understanding of how masks would perform in a clinical setting in terms of fit and seal, and whether elastic straps or nose bridge would be damaged or decontaminated. Most decontamination studies did not assess mask fit. There were no decontamination studies that evaluated clinical outcomes, such as rate of infection among healthcare providers. In addition, even the more promising approaches remain theoretical, as none of the preclinical studies tested decontamination for the SARS-CoV-2 pathogen. Proxy measures such as MS2 bacteriophages and aerosolised sodium may not be generalisable to the SARS-CoV-2 pathogen.
None of the clinical research occurred during an actual pandemic/epidemic setting, and studies assessing user compliance and discomfort may not be generalisable to such scenarios. As interventions were tested in highly controlled environments, they may not be generalisable to an outbreak setting, in which there may be system-wide disorganisation, resource overload, extended use times and limited personnel.
Our findings align with the current research base. There has been significant interest in pandemic preparedness, including cost-benefit analyses of stockpiling, methods to conserve ventilators, infection control modelling and strategies to improve surge capacity.88–91 In previous outbreaks such as Ebola and influenza, hospital leaders have noted the importance of rapid PPE acquisition in response to sudden spikes in demand.92 93 However, such efforts can fail to meet demand in times of pandemic, such as with COVID-19. In addition, willingness of health providers to work during pandemics is associated with their perception of safety.94–97 Absenteeism may cause reduction in surge capacity or even basic staffing if there are mask shortages for providers.94–96 98 The need to conserve available PPE for healthcare providers during the COVID-19 pandemic has informed guidelines for PPE use in lower risk groups, such as asymptomatic community members, and prompted research priorities regarding decision-making, such as whether surgical masks are as effective against COVID-19 as N95 respirators.99–101
Strengths of our systematic scoping review included a robust search of the literature after consultation with a research librarian. This included further hand search of citations of included articles and reviews, and a search of grey literature, including preprint databases. We undertook duplicate screening, extraction and evidence grading by at least two independent reviewers. Limitations include the restriction of examined studies to those published in English and to the last 25 years. Furthermore, we were limited to the quality of the evidence base in the search yield.
The US Food and Drug Administration (FDA) issued a guidance in May 2020 to provide recommendations for sponsors of decontamination and bioburden reduction systems about what information should be included in a pre-emergency use authorisation (pre-EUA) and/or EUA request to help facilitate FDA’s efficient review of such request.102 This policy was intended to remain in effect only for the duration of the COVID-19 pandemic. As this guidance was issued subsequent to design and execution of the studies we have reviewed, we did not seek to measure their published results retroactively against the FDA guidelines. Future studies aimed at respirator conservation (including decontamination, reuse and use beyond manufacturer’s expiry date) should consider these guidelines during protocol design.
Ultimately, we recommend further clinical research on mask conservation strategies, both in the current COVID-19 context as well as in preparation for any future disease outbreaks. Higher quality research, especially RCTs, is necessary for determining whether mask conservation strategies are effective against the SARS-CoV-2 pathogen specifically. While deviations from standard of care may be necessary in times of PPE shortage, it is important that evidence-informed decisions are made for both patient and provider safety.
Conclusion
Promising strategies for mask conservation in the context of pandemics and epidemics include use of stockpiled masks, extended wear of disposable masks, and UV-based methods for decontamination. Strategies that were found to be less effective included the use of cloth masks, layering multiple surgical masks and re-donning previously used respirators. However, there remains uncertainty regarding the effectiveness of these strategies in a clinical setting, as well as their generalisability to COVID-19. Further research is needed prior to clinical implementation.
Acknowledgments
Thank you to Kaitlin Fuller from Gerstein Science Information Centre, University of Toronto for her assistance and guidance in creation of the search strategy.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @DrJBryan
Contributors AK, JMB and SMF conceived the study. SMF supervised the conduct of the scoping review and data collection. AK, SK, TG and MY drafted the abstract and completed full-text screening, data analysis and grading. JMB and SMF addressed any discrepancies and validated results. AK, JMB and SMF drafted the manuscript, and all authors contributed substantially to its revision.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplemental information. Data are available on reasonable request.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.