Abstract
Targeted deep sequencing across broad genomic regions has been used to detect circulating tumor DNA (ctDNA) in pancreatic ductal adenocarcinoma (PDAC) patients. However, since most PDACs harbor a mutation in KRAS, sequencing of broad regions needs to be systemically compared to analyzing only KRAS mutations for PDAC. Using capture-based targeted deep sequencing, we detected somatic tumor mutations in 17 fine needle aspiration biopsy and 69 longitudinal cell-free DNA (cfDNA) samples from 17 PDAC patients. KRAS mutations were detected in 10 out of 17 pretreatment patient plasma samples. Next, interrogation of genetic alterations in matched primary tumor samples detected ctDNA in 12 of 17 pretreatment plasma samples and cfDNA sequencing across the 83 target genes identified ctDNA in 15 of 17 cases (88.2% sensitivity). This improved sensitivity of ctDNA detection resulted in enhanced tumor burden monitoring when we analyzed longitudinal plasma samples. We found that cfDNA sequencing detected the lowest mutant allelic fractions and number of variants when complete response or partial response to chemotherapy was achieved. We demonstrated that ctDNA levels measured by targeted deep sequencing sensitively indicate the presence of cancer and correlate well with clinical responses to therapy and disease progression in PDAC patients.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading mortality-causing diseases internationally, which is due, at least in part, to the lack of a noninvasive biomarker for sensitive and specific disease detection1. Because a potentially curative operation that facilitates long-term survival is primarily successful for patients with the clinically localized disease2,3, numerous studies have attempted to identify a highly accurate blood-based biomarker for early detection of PDAC. Nonetheless, cancer antigen (CA) 19-9 remains the standard biomarker4, despite its unsatisfactory sensitivity and specificity for early detection of disease5, which also limits its role monitoring disease burden. Recently, circulating tumor DNA (ctDNA) has been proposed as an alternative to traditional noninvasive protein biomarkers due to its potential for use in a wide range of clinical applications for various cancers, including PDAC6,7,8. Because ctDNA is released from tumor cells into the blood, the presence of ctDNA, as detected through mutations harbored by the original tumor, is indicative of a tumor and relative tumor burden7,9. Previous studies have reported highly sensitive and specific genetic profiling of plasma DNA, suggesting that the use of ctDNA as a liquid biopsy might significantly improve current systems of tumor diagnosis10, tumor progression monitoring9, targeted therapies11, and early-stage detection12. While expected to revolutionize cancer diagnoses in general13, liquid biopsy based on ctDNA analysis is even more anticipated for particular cancer types, such as PDAC, where biopsy is risky and often untenable.
Among the various methods of detecting ctDNA, digital PCR methods, including BEAMing (beads, emulsion, amplification, and magnetics), have been effectively utilized to detect a limited number of specific target variants, including KRAS, EGFR, and PIK3CA hotspot mutations, across various cancers7,11,14,15,16,17,18. Because mutations in KRAS are observed in >90% of PDAC1 and are likely to be clonal mutations present in the majority of cancer cells, they are often identified in plasma as a ctDNA benchmark for PDAC6,18,19,20,21,22. This unique mutational feature of PDAC renders digital PCR very attractive for ctDNA detection in PDAC patients via interrogating a few KRAS hotspots6,8,18,20.
Despite the considerably high sensitivity of digital PCR, the detection of KRAS mutations in plasma using this method has often fallen short of high expectations, as the ctDNA detection rate has averaged as low as 50%6,19,20,21,23. This limitation may be a result of there being low allelic fractions of KRAS mutations in a subset of PDACs24. In fact, the allelic fractions of KRAS mutations in PDAC biopsy samples range from homozygous wild-type to ≥100% mutated KRAS, indicating KRAS-mutated populations might be subclonal in a significant fraction of PDAC patients1,24.
Targeted deep sequencing has been employed to interrogate tumor variants across relatively broad genomic regions that include many cancer-associated target genes using blood samples from patients with various types of cancer9,10,22,25,26. It is now beyond doubt that ctDNA sequencing analysis of broad genomic regions facilitates evaluations of the tumor burden25,27, intra-tumor genetic heterogeneity28, emergence of resistant mutations29,30, and clonal expansion31 during disease progression. Conversely, interrogation of broad genomic regions requires more resources for generation of raw data and subsequent downstream analysis. Additionally, it might generate more frequent false positives32, unless detection sensitivity is compromised to a certain degree. Therefore, deep sequencing dozens to hundreds of cancer-related genes have to be carefully evaluated to determine if the benefits outweigh the disadvantages, especially for PDAC, where at least one of a few KRAS variants are observed in most cases. Here, we evaluated the benefits of investigating 83 target genes to detect ctDNA in pancreatic cancer patients and compared the method to testing either KRAS hotspots or genetic variants in their matched biopsy samples.
Results
Generation of targeted deep sequencing data for PDAC patients
To evaluate ctDNA detection by targeted deep sequencing and its clinical utilities, 17 PDAC patients with available tumor biopsy samples underwent blood draws for cell-free DNA (cfDNA) testing (Table 1). We profiled a total of 120 samples from these 17 patients consisting of 17 fine needle aspiration (FNA) biopsies, 34 peripheral blood leucocyte (PBL) samples, and 69 plasma samples (Supplementary Fig. S1 and Table S1). Of this sequencing data, 17 plasma and 17 PBL samples obtained prior to treatment were recently published in our study analyzing sequencing background noise33.
To achieve a mean sequencing depth of ~10,000X (prior to duplicate removal) in a cost-effective manner, we designed a pool of RNA baits targeting 83 cancer-associated genes, including hotspots for pancreatic cancer (Supplementary Table S2). DNA libraries were constructed from plasma DNA and matched germline DNA (i.e., PBL genomic DNA) samples and sequenced on Illumina HiSeq. 2500 as we previously reported33. After excluding PCR duplication, the unique coverage depths for the biopsy, plasma DNA, and germline DNA samples averaged 987.15X (790.32–1476.55X), 2227.25X (490.27–5532.04X), and 1928.55X (1042.12–2843.48X), respectively (Supplementary Table S1).
From these data sets, we detected mutations in FNA biopsies (MFNA) and plasma samples (MP) as described below. Hereafter, depending on the method of detecting MP, the following notations will be used: MP/KRAS, mutations detected in KRAS; MP/FNA, mutations detected in MFNA; MP/TR-BF, mutations detected in target regions in a biopsy-free manner; MP/TR, mutations detected in target regions combined with MP/FNA and MP/TR-BF.
KRAS mutations
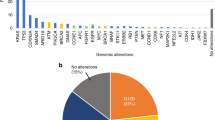
First, because most PDACs carry a KRAS mutation, we determined whether these mutations were present in 17 pretreatment patient samples. We detected KRAS mutations in 10 plasma (58.8%) and 13 FNA samples (76.5%) (Fig. 1). Because the allelic fractions of somatic variants are usually notably lower in plasma samples than in tissue samples, the lower detection rate of KRAS mutations in plasma samples than in FNA samples was the expected result. The mean ± standard error of the mean (SEM) of KRAS mutant allele frequencies (MAFs) were 21.18% ± 4.06 in FNA samples and 2.02% ± 0.67 in plasma samples.
Detection of ctDNA in pretreatment plasma samples from 17 pancreatic cancer patients. The top panel summarizes the mutations detected in the 17 patients based on method (i.e., MP/KRAS, MP/FNA, and MP/TR). While interrogation of KRAS hotspots (MP/KRAS) detected ctDNA from 10 patients, analyses of variants detected from FNA samples (MP/FNA) and broad genomic target regions (MP/TR) detected tumor variants in 12 and 15 plasma samples, respectively. The oncoprint chart presents the MFNA and MP/TR at the gene level. If a variant was detected in both the MFNA and MP/TR, the variant also corresponded with MP/FNA. The number of affected genes for each patient was plotted at the bottom of the chart. The number of samples that harbored a mutation in each gene is plotted on the right side of the chart. Two * and four † independent mutations in PDGFRB and ATM were detected in the P43 FNA and plasma samples, respectively.
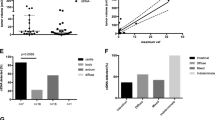
To exclude the possibility that the moderate MP/KRAS (plasma DNA mutations in KRAS) detection rate was due to poor analytic sensitivity of the method, we evaluated the analytic detection sensitivity and specificity of the targeted deep sequencing method for KRAS mutations using 62 consecutive samples from 14 patients. Based on droplet digital PCR (ddPCR) analysis of the samples, the targeted deep sequencing method had a 95.7% sensitivity with a 95% confidence interval (CI) of 78.1 to 99.9% and a 100% specificity with a 95% CI of 91.2 to 100% for detection of KRAS mutations (Supplementary Table S3 and S4), thus negating the possibility of poor sensitivity.
Therefore, evaluating only MP/KRAS may be limited by the moderate sensitivity of the method for detecting ctDNA, despite the majority of PDAC patients carrying KRAS mutations.
Detection of single nucleotide variants (SNVs) across broad genomic target regions
Utilizing customized capture-based targeted sequencing, we detected a total of 40 mutations in FNA samples (MFNA) from 17 patients, including 2 specimens from patients P11 and P28 who had no significant mutations (Supplementary Table S5). In order to detect ctDNA, the 40 MFNA were statistically evaluated to determine the significance of these mutations in the matched pretreatment plasma DNA samples (p < 0.001). Among the 40 mutations found in the FNA samples, 28 were detected in the corresponding plasma samples (MP/FNA) and were significantly above the background noise of the plasma DNA, resulting in a 70.0% detection sensitivity with an average MAF of 1.60% ± 0.31 (mean ± SEM) (Fig. 1a and Supplementary Table S6).
Next, we attempted to detect ctDNA by analyzing broad genomic target regions in a biopsy-free manner (MP/TR-BF) as described in previous studies with a minor modification (Materials and Methods)9,34. This approach aimed to detect somatic variants without profiling tissue samples, eschewing the necessity of biopsy. From this analysis, we detected 27 MP/TR-BF in pretreatment plasma samples, including 15 mutations concordantly detected in FNA samples (Fig. 1b and Supplementary Table S6) with a median MAF of 3.54% ± 1.38. Twelve mutations were detected in the plasma DNA samples, but not in the matched FNA biopsy samples.
From the MP/TR-BF detected in the plasma samples either prior to treatment or peri/post-treatment, but not in their matched FNAs, we selected two variants (ROS1 p.I1967V and RB1 p.251X) from two patients (P2 and P5) and performed ddPCR to validate their presence in 8 plasma DNA samples (Supplementary Table S3). Consistent with the cfDNA sequencing results, these mutations were detected in consecutive plasma samples from these patients, indicating that variants detected only in plasma were unlikely to be false positives.
To estimate the frequency of false positives from the technical background noise, we generated sequencing data for biological replicates (n = 21) using PBL DNA from six patients. One of the replicates from each patient was paired with the other as a mock-matched plasma sample and was processed for variant detection as described in Materials and Methods. No variants were detected out of a total of 21 replicates, indicating a minimal false discovery rate due to technical background. Collectively, our results show that targeted deep sequencing of plasma DNA in biopsy-free situations is a feasible means of detecting tumor-specific somatic variants across target regions.
There are limitations to using FNA samples for genetic profiling as they do not sufficiently represent all regional subclonal events. Our data suggests that somatic profiling of mutations in plasma DNA in a biopsy-free manner compensates for the shortcomings of FNA samples, revealing intra-tumor heterogeneity. Based on mutations in plasma DNA in target regions (MP/TR) combined with MP/FNA and MP/TR-BF, we were able to detect ctDNAs in 15 out of 17 pretreatment samples, suggesting there is a significant advantage to profiling broader genomic regions instead of KRAS hotspots.
Monitoring tumor burden by measuring ctDNA
We next examined whether ctDNA levels correlated with patient clinical response to therapy and disease progression. First, we measured cancer antigen 19-9 (CA 19-9) and MP/TR in consecutive blood draws using separate draws for each type of test from ten PDAC patients undergoing distinct treatment protocols. Except for two patients (P11 and P27), where variants were not detected in either primary or plasma samples, the data from the remaining eight patients is displayed in Fig. 2. Next, we examined if the MP/TR frequencies and CA19-9 levels were indicative of changes in disease burden in these eight patients, in particular in terms of evaluation of complete response/partial response (CR/PR) and progressive disease (PD). MP/TR were not detected in all five cases (P5, P7, P23, P31, and P43) when PR was determined and were elevated in all six cases (P2, P5, P7, P36, P42, and P43) when PD was observed. Therefore, these values positively correlated with disease burden in all 11 patients (Supplementary Table S7). Meanwhile, CA19-9 levels properly signaled a positive response in two out of four cases of PR and disease progression in four out of six cases of PD (Supplementary Table S7). These data suggest that ctDNA detected by MP/TR is a better surrogate marker of patient response to chemotherapy and/or disease progression than CA19-9 in PDAC patients.
Monitoring of ctDNA in pancreatic cancer patients undergoing therapeutic interventions. The ctDNA levels estimated by MP/TR were plotted on the left y-axis for eight patients (a–h). The entire list of MP/TR is summarized in Supplementary Table 6. Chemotherapeutic agents administered to each patient and therapeutic response was evaluated based on the Response Evaluation Criteria In Solid Tumors are displayed at the top of the graph. CA 19-9 level (yellow solid line) and tumor size (dark-khaki dotted line) based on CT images were plotted against the right y-axis. CCRT, concurrent chemoradiation therapy; CR, complete response; DOD, dead of disease; Dx, diagnosis; E, erlotinib; GEM, gemcitabine; FU, fluorouracil; P, cisplatin; Met, metastasis; ND, not detected; Op, operation; PD, progressive disease; PR, partial response; SD, stable disease.
Among the eight patients, five patients (P5, P7, P23, P31, and P43) had computed tomography (CT) imaging data available more than three times during the tracing of the MP/TR level. For three of these patients (P23, P31, and P43), significant decreases in the MP/TR levels were followed by a PR evaluation (Fig. 2d,e,h). On the other hand, significantly detectable ctDNA levels and/or dramatic increases in the MP/TR level were followed by disease progression in P5 and P43 patients (Fig. 2b,h). On average, alterations in MP/TR frequencies were detected 2 months ahead of the CT imaging changes observed in these patients, suggesting that ctDNA measured by targeted deep sequencing is the earliest indicator of disease status. Taken together, these results suggest there is clinical utility for cfDNA sequencing in monitoring of PDAC patient clinical responses to therapy and disease progression.
Next, to compare the allelic frequency of ctDNA with tumor burden, we divided our ctDNA data into four groups based on ctDNA at the time of 1) diagnosis (Dx) and when 2) CR/PR, 3) stable disease (SD), and 4) PD were noted (Fig. 3). The average allelic frequencies of ctDNA measured by all three different approaches varied among the groups, where the most significant was for MP/TR (analysis of variance (ANOVA), least significant difference (LSD), p-value = 4.5 × 10−9, Fig. 3c) followed by MP/KRAS (ANOVA, LSD, p-value = 0.0018, Fig. 3a). We noticed the allelic fractions of MP/TR near the time of CR/PR were significantly lower than those at the time of Dx, SD, and PD (Fig. 3c). Because an increase in MP/TR frequency was likely to precede determination of PD, we moved data points obtained less than 3 months before PD, regardless of what group they belonged to, into the PD group in order to take the time lag into account. When we made this adjustment, the statistical significance increased further, supporting this finding (ANOVA, LSD, p-value = 1.2 × 10−12, Supplementary Fig. S2). Meanwhile, CA 19-9 levels were not significantly different among patients with different disease statuses (ANOVA, LSD, p-value = 0.13, Fig. 3d).
Correlation of allelic frequency of ctDNA and CA19-9 levels with patient response to therapy. The allelic frequencies of (a) MP/KRAS, (b) MP/FNA, and (c) MP/TR were box-plotted based on evaluations of their near-time response to therapy and diagnosis. (d) CA 19-9 levels were box-plotted. All of the determined levels are presented on a logarithmic scale. In each box-plot, median and mean are indicated by a horizontal bar and red diamond, respectively. The level of statistical significance (ANOVA, LSD) is indicated as *P ≤ 0.05, **P ≤ 0.001, and ***P ≤ 0.00001. CR, complete response; Dx, diagnosis; PD, progressive disease; PR, partial response; SD, stable disease.
Because the allelic frequencies in ctDNA varied according to disease status, we also quantified the average number of variants detected per sample (Fig. 4). We noticed the number of variants detected differed significantly among patients with different disease statuses when analyzing MP/FNA (Fig. 4a, ANOVA, LSD, p-value = 1.8 × 10−5) and MP/TR (Fig. 4b, ANOVA, LSD, p-value = 5.7 × 10−8). Similar to the diminished allelic frequencies of ctDNA in the CR/PR group, the number of MP/FNA and MP/TR had significantly decreased at the time of CR/PR compared to the other groups. To exclude the possibility that the low allelic frequencies and numbers of MP/TR in the CR/PR group were due to technical artifacts, we compared sequencing metrics among the groups. We found no differences in these parameters, thus ruling out the aforementioned possibility (Supplementary Figs S3 and S4).
Number of mutations in plasma DNA. The (a) MP/FNA and (b) MP/TR in each sample were box-plotted according to evaluations of near-time therapy response and diagnosis. The level of statistical significance (ANOVA, LSD) is indicated as *P ≤ 0.05, **P ≤ 0.001, and ***P ≤ 0.00001. CR, complete response; Dx, diagnosis; PD, progressive disease; PR, partial response; SD, stable disease.
Noticeably, the allelic frequency and number of MP/TR started to decrease immediately after treatment was initiated and were lowest around 4 months of post-treatment (Fig. 5). Thereafter, both the allelic frequency and number of MP/TR significantly increased as treatment duration increased (ANOVA, LSD, p-value = 0.008 for Fig. 5a; ANOVA, LSD, p-value = 0.004 for Fig. 5b). These data are also consistent with our results showing that MP/TR correlates with evaluation of response to therapy, as PR was most frequently observed around up to 4 months following treatment with chemotherapeutics.
Overall, our results indicate that MP/TR is a better representation of real-time disease status based on either allele frequency and/or number of mutations than MP/KRAS and MP/FNA. Taken together, our results strongly suggest that the allelic and/or MP/TR frequencies are good indicators of real-time disease status in PDAC patients, providing information not only concerning the presence of disease, but also whether there is a positive response to therapy, even when it is a partial response.
Discussion
In this study, we evaluated ctDNA-based approaches for detecting PDAC. Specifically, we profiled (1) somatic variants in KRAS hotspots (MP/KRAS), (2) patient-specific mutations in tissue samples (MP/FNA), and (3) broad genomic target regions (MP/TR). Of these approaches, we found comprehensive analysis of ctDNA across broad genomic target regions was the most sensitive at detecting PDACs and accurately monitoring tumor burden. Furthermore, analysis of ctDNA might overcome the limitation in variant detection in FNA samples due to tumor purity and/or intra-tumor heterogeneity.
Among the 17 pretreatment plasma samples, we detected ctDNA in ten, twelve, and fifteen samples when profiling MP/KRAS, MP/FNA, and MP/TR, respectively, indicating an increased sensitivity of cancer detection by analyzing cfDNA across broad genomic target regions. Moreover, this improved sensitivity in ctDNA detection enhanced tumor monitoring by longitudinal cfDNA analysis. For instance, in patients P5 and P42, although a KRAS mutation was not detected in pretreatment samples or any subsequent peri/post-treatment samples, multiple independent variants in other genes were detected whose levels consistently correlated with tumor burden (Fig. 2b,g). In patient P2, ROS1 p.I1967V levels in plasma DNA dramatically decreased after a surgical operation, which is indicative of tumor removal; however, this was not reflected in the MP/KRAS and MP/FNA (Fig. 2a).
Despite its advantages, interrogation of broader genomic regions may result in a higher frequency of false positives, especially when performed in a biopsy-free manner. In fact, several studies have utilized digital PCR in parallel with targeted deep sequencing, which can complement or cross-validate each other for detecting ctDNA in various types of cancers8,35,36. Furthermore, the size of the target regions queried has been taken into account for optimally balancing the sensitivity and specificity of detecting ctDNA in previous studies9,32. To minimize these false positives, we used stringent filtering steps when calling MP/TR-BF. Then, we evaluated if the filtering steps during variant calling were adequate for reducing the rate of false discoveries. By analyzing duplicated PBL genomic DNA sequencing data, we found that false positives due to technical background were minimal, as described in the Results section. In addition, we validated some of the MP/TR-BF not detected in FNA specimens by ddPCR, which indicated using the variant detection method in a biopsy-free manner was stringent enough to remove most false positives.
The sensitivity of variant detection is significantly dependent on the depth of unique coverage, although this is not the sole determinant. Since the method to call MP/TR-BF did not use a threshold of the MAF, we calculated the lowest MAF of detectable MP/TR-BF, which varied depending on the depth of unique coverage (Supplementary Fig. S5a). In addition, we examined the allele-specific background error rates across the target regions (Supplementary Fig. S5b) because a practical limit of detection is also imposed by errors introduced during sample preparation and sequencing. However, due to elevated background error rates, only 1.58% of alternative alleles required more supporting reads than the threshold (n = 8) in the calling algorithm for MP/TR-BF (Supplementary Fig. S5c). These data indicated that the lowest theoretical MAF was applicable to >98% of the alternative alleles. We next calculated the lowest theoretical MAF of MP/TR-BF depending on the amount of input DNA, which was correlated with the depth of unique coverage as shown in Supplementary Fig. S6a. The average threshold for calling MP/TR-BF varied in the range of 0.24–0.70% depending on the input DNA amount (7.8–102 ng, except for one outlier of 212 ng) used for library construction in this study.
When we sequenced cfDNA reference DNA (50 ng) from Horizon, which contains hotspot mutations at a frequency of 0.1%, 1%, and 5%, we were able to detect all six SNVs at the 1% frequency but none at the 0.1% frequency using the MP/TR-BF calling method. The data were consistent with the estimated lowest theoretical MAF of MP/TR-BR. Although the number of variants tested was small and the interval of tested frequency was wide, the data showed that the MAF detection limit of the method was around 1% or less.
Because we applied stringent filtering steps to minimize the false discovery rate when detecting MP/TR-BF, the variant calling method might compromise the analytic detection sensitivity. Combining this method with MP/FNA allowed detection at a high sensitivity by taking advantage of patient-specific mutations in tissue samples. Therefore, we could compensate for the compromised detection sensitivity while interrogating somatic tumor variants across broad genomic regions.
Molecular barcoding has recently been shown to minimize technical background noise and overcome the loss of unique reads due to fragment identification collision during deduplication26. However, this method was not utilized in this study. Therefore, modification to include molecular barcoding is anticipated to further improve the specificity and sensitivity of targeted deep sequencing of broad genomic regions to detect ctDNA.
As the profiles of genetic alterations in PDAC may allow physicians to determine tailored therapeutics by offering useful information on probable clinical outcomes, genetic testing using tumor samples is becoming increasingly important37. Although genetic tests for PDAC are often conducted using endoscopic ultrasound (EUS)-guided FNA samples38,39, FNA specimens often have low cellularity and contain a significant fraction of normal stromal cells24. Consequently, genetic tests using FNA samples frequently suffer from small input amounts of DNA that contain only a small fraction of tumor DNA, which affects the analytic detection sensitivity of these tests40. We also detected KRAS mutations (allele frequency ≥4%) in 76.5% of FNA specimens, which is somewhat lower than KRAS mutation-positive rates observed in surgical specimens. Additionally, FNA samples might not thoroughly represent various subclonal populations in heterogeneous tumor masses. As our results included tumor variants that were not detected in FNA samples, but were detected in the corresponding plasma samples, the limitations of using FNA samples for genetic testing may be at least partly overcome by detecting ctDNA by analyzing broad genomic regions.
There are obvious hurdles for translating targeted sequencing of cfDNA into clinical tests for PDAC. First, the higher cost of targeted deep sequencing might outbalance the benefit of profiling genetic alterations across broad genomic regions at this time. However, the recent dramatic reduction in sequencing costs implies that the cost-effectiveness of this method is likely to increase in the near future. Second, this study is a pilot test that analyzed only a small number of cases with the heterogeneous treatments, although we revealed the advantages of analyzing broad genomic regions to detect ctDNA in PDAC patients. A large cohort study is required to evaluate the clinical utility of this method for PDACs.
In summary, our results suggest that using targeted deep sequencing of broad genomic regions to detect ctDNA has diagnostic advantages, even for PDACs that typically harbor a KRAS hotspot mutation.
Materials and Methods
Patient samples
The institutional review board at the Samsung Medical Center approved this present study (IRB number 2014-04-048-009) and all the methods were carried out in accordance with the approved guidelines. Written informed consent was obtained from all subjects. Newly diagnosed PDAC patients who had undergone EUS-guided FNA were enrolled in and underwent blood draws for cfDNA testing. The pretreatment (i.e., before treatment) blood draw of participants was collected at the time of diagnosis.
Plasma and PBL sample preparation
Whole blood samples were collected in Cell-Free DNA™ BCT tubes (Streck Inc., Omaha, NE, USA). Plasma was prepared using 3 centrifugation steps with increasing centrifugal force: 840 × g for 10 min, 1040 × g for 10 min, and then 5000 × g for 10 min at room temperature. PBLs were collected from the initial centrifugation step. Collected plasma and PBL samples were stored at −80 °C until DNA extraction.
DNA sample preparation
PBL genomic DNA was isolated using a QIAamp DNA mini kit (Qiagen, Santa Clarita, CA, USA). Plasma DNA was obtained from 2 to 5 mL of plasma using a QIAamp Circulating Nucleic Acid Kit (Qiagen). An AllPrep DNA/RNA Mini Kit (Qiagen) was used to purify genomic DNA from FNA tissues. The concentration, purity, and fragment size of DNA were assessed as previously described33,41. In addition, the Horizon cfDNA reference standard set (HD780, Horizon Discovery Group plc, Cambridge, UK) was obtained to evaluate the performance of variant detection. SNVs in the reference materials included EGFR L858R, EGFR T790M, KRASG12D, NRAS Q61K, NRAS A59T, and PIK3CA E545K.
Library preparation
Purified genomic DNA was sonicated (7 min, 0.5% duty, intensity of 0.1, and 50 cycles/burst) into 150–200 bp fragments using a Covaris S2 (Covaris Inc. Woburn, MA, USA). The FNA sample libraries were constructed using the SureSelect XT reagent kit, HSQ (Agilent Technologies) according to the manufacturer’s instructions. The PBL and plasma DNA libraries were created using a KAPA Hyper Prep Kit (Kapa Biosystems, Woburn, MA, USA) as described previously33,41. When constructing the sequencing libraries, 200 ng of PBL DNA and 37.12 ng of plasma DNA were used on average. Briefly, after end repair and A-tailing according to the manufacturer’s protocol, we performed adaptor ligation at 4 °C overnight using a pre-indexed PentAdapter™ (PentaBase ApS, Denmark). After amplification using 9 PCR cycles, the library was analyzed for quantity and fragment size distribution and then underwent multiplexing hybrid selection to enrich for targets. Hybrid selection was performed using customized RNA baits that targeted ~499 kb of the human genome, including exons from 83 cancer-related genes (Supplementary Table 1). Up to 8 purified libraries were pooled and a total of 750 ng of each pooled library was used for hybrid selection reactions. Target enrichment was performed according to the SureSelect (Agilent Technologies) bait hybridization protocol except the blocking oligonucleotide was replaced with the IDT xGen blocking oligonucleotide (IDT, Santa Clara, CA, USA) as the pre-indexed adapter. After target enrichment, the captured DNA fragments were amplified using 13 cycles of PCR with oligonucleotides P5 and P7.
Sequencing and data processing
Based on DNA concentration and average fragment size, each library was diluted to a concentration of 2 nM and pooled in equal volumes. The libraries were denatured using 0.2 N NaOH, diluted to 20 pM in hybridization buffer (Illumina, San Diego, CA, USA), and then subjected to cluster amplification according to the manufacturer’s protocol (Illumina). Flow cells were sequenced in the 100 bp paired-end mode using HiSeq. 2500 v3 Sequencing-by-Synthesis Kits (Illumina) and then analyzed using RTA v.1.12.4.2 or later. All of the raw data were aligned to the hg19 human reference and BAM files were created using BWA-mem (v0.7.5)42. SAMTOOLS (v0.1.18)43, Picard (v1.93), and GATK (v3.1.1)44 were used for sorting SAM/BAM files, local realignment, and duplicate markings, respectively. We filtered reads to remove duplicates, discordant pairs, and off-target reads as previously described33.
SNV detection in FNA samples and statistical tests for the SNVs in the matched plasma
For FNA biopsy specimens, somatic SNVs were detected using MuTect 1.1.445 and Varscan246 with matched germline (i.e., PBL) samples. For Varscan2, the default parameter values were used with previously described modifications9. Somatic SNVs identified by at least one method were retained if they were present at a frequency lower than 0.5% in the matched PBL sample and higher than 4% in the tumor sample. Somatic SNVs found in the FNA samples (MFNA) were listed and tested for their presence in the paired plasma samples as described previously9 in our recent study34. After background alleles in each sample were adjusted based on position-specific error rates, it was determined if the allelic frequency of a given SNV fell within the 95th percentile of the adjusted background alleles. The depth of unique coverage, strand bias, and supporting read count were also considered for SNV detection.
Biopsy-free SNV (MP/TR-BF) identification in plasma DNA
A method from previous studies8,9 was modified slightly to identify somatic SNVs in plasma samples as described in our recent study34. First, positions with a strand bias under 0.9 and total read depth over 500 were considered for analysis. All non-reference alleles were subjected to Phred quality filtering using a threshold Q of 30. Non-reference alleles present at a frequency lower than 0.5% in the matched germline DNA were subjected to the binomial test to determine if a non-reference allele was significantly more abundant in plasma DNA than the matched germline DNA. Multiple testing adjustments were made using the Bonferroni correction. Next, z-tests were performed to compare the frequencies of nonreference alleles to their background allele frequency distribution in other plasma DNA samples. For comparison, a background allele frequency distribution was generated by selecting non-reference alleles in plasma DNA present at a frequency <2.5% in the paired tumor and <0.5% in the paired germline DNA with a sufficient total depth (≥250× in tumor tissue, ≥500× in PBL, and ≥500× in plasma). In addition, the following filters were applied: (1) candidate alleles with less than eight supporting reads, (2) all candidates with an allele frequency <20% when there were two or more candidates within any 10 bp window, and (3) candidates with a Bonferroni adjusted p-value higher than 10−18 from the z-test were discarded. To remove potential false positives due to cross-contamination among multiplexed samples, we excluded SNV candidates if they were found as germline single nucleotide polymorphisms (SNPs) in other samples processed in the same lane of a sequencing flow cell. Nonsynonymous, stop-gain, stop-loss, and splicing-disrupting variants were listed as the final positive calls.
Droplet digital PCR validation
Mutant and wild-type alleles in plasma samples were quantified using the QX200 Droplet Digital PCR System (Bio-Rad, Hercules, CA, USA). TaqMan assays for KRAS p.G12D/G12V were obtained from Bio-Rad (PrimePCR ddPCR Mutation Assay) and RB1 p.R251* and ROS1 p.I1967V assays were custom generated by TaqMan SNP Genotyping Assays (Thermo Fisher Scientific, Waltham, MA, USA). The concentrations of wild-type and mutant DNA (copies per µl) in each sample were calculated using the manufacturer’s software and the concentrations in plasma (copies per mL) were derived as described in van Ginkel, J.H et al.47.
Statistical analysis
To evaluate whether the means between multiple groups were significantly different, we used one-way ANOVA with LSD post-hoc analysis. For all of the tests, statistical significance was set at 5% and reported as two-tailed p-values. Statistical analyses were carried out using PASW Statistics for Windows version Release 18.0.0 (formerly SPSS, IBM Corporation, Armonk, New York).
Accession codes
Raw sequencing data were deposited in the Sequence Read Archive with accession number SRP097813.
References
Makohon-Moore, A. & Iacobuzio-Donahue, C. A. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer 16, 553–65 (2016).
Kubota, M. et al. Cancer chemotherapy and somatic cell mutation. Mutat Res 470, 93–102 (2000).
Ryan, D. P., Hong, T. S. & Bardeesy, N. Pancreatic adenocarcinoma. N Engl J Med 371, 1039–49 (2014).
Lennon, A. M. & Goggins, M. Diagnostic and Therapeutic Response Markers. In Pancreatic Cancer 675-701 (Springer New York, New York, NY, 2010).
Takai, E. & Yachida, S. Circulating tumor DNA as a liquid biopsy target for detection of pancreatic cancer. World J Gastroenterol 22, 8480–8488 (2016).
Pietrasz, D. et al. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients Is a Prognostic Marker. Clin Cancer Res 23, 116–123 (2017).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6, 224ra24 (2014).
Takai, E. et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci Rep 5, 18425 (2015).
Newman, A. M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 20, 548–54 (2014).
Murtaza, M. et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat Commun 6, 8760 (2015).
Remon, J. et al. Osimertinib benefit in EGFR-mutant NSCLC patients with T790M-mutation detected by circulating tumour DNA. Ann Oncol 28, 784–790 (2017).
Phallen, J. et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med 9 (2017).
Wan, J. C. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer (2017).
Diehl, F. et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 14, 985–90 (2008).
Oxnard, G. R. et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 34, 3375–82 (2016).
Sacher, A. G. et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol 2, 1014–22 (2016).
Li, M., Diehl, F., Dressman, D., Vogelstein, B. & Kinzler, K. W. BEAMing up for detection and quantification of rare sequence variants. Nat Methods 3, 95–7 (2006).
Tjensvoll, K. et al. Clinical relevance of circulating KRAS mutated DNA in plasma from patients with advanced pancreatic cancer. Mol Oncol 10, 635–43 (2016).
Dabritz, J., Preston, R., Hanfler, J. & Oettle, H. Follow-up study of K-ras mutations in the plasma of patients with pancreatic cancer: correlation with clinical features and carbohydrate antigen 19-9. Pancreas 38, 534–41 (2009).
Brychta, N., Krahn, T. & von Ahsen, O. Detection of KRAS Mutations in Circulating Tumor DNA by Digital PCR in Early Stages of Pancreatic Cancer. Clin Chem 62, 1482–1491 (2016).
Ako, S. et al. Utility of serum DNA as a marker for KRAS mutations in pancreatic cancer tissue. Pancreatology 17, 285–290 (2017).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science (2018).
Earl, J. et al. Circulating tumor cells (Ctc) and kras mutant circulating free Dna (cfdna) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer 15, 797 (2015).
Cancer Genome Atlas Research Network. Electronic address, a.a.d.h.e. & Cancer Genome Atlas Research, N. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 32, 185–203 e13 (2017).
Forshew, T. et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med 4, 136ra68 (2012).
Newman, A. M. et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol 34, 547–55 (2016).
Dawson, S. J. et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 368, 1199–209 (2013).
De Mattos-Arruda, L. et al. Capturing intra-tumor genetic heterogeneity by de novo mutation profiling of circulating cell-free tumor DNA: a proof-of-principle. Ann Oncol 25, 1729–35 (2014).
Murtaza, M. et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497, 108–12 (2013).
Vandekerkhove, G. R. et al. Circulating tumor DNA reveals clinically-actionable somatic genome of metastatic bladder cancer. Clin Cancer Res (2017).
Abbosh, C. et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545, 446–451 (2017).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Park, G. et al. Characterization of background noise in capture-based targeted sequencing data. Genome Biol 18, 136 (2017).
Kim, J. Y. et al. Circulating tumor DNA shows variable clonal response of breast cancer during neoadjuvant chemotherapy. Oncotarget 8, 86423–86434 (2017).
Siravegna, G. et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med 21, 795–801 (2015).
Leary, R. J. et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med 2, 20ra14 (2010).
Schneider, G., Schmidt-Supprian, M., Rad, R. & Saur, D. Tissue-specific tumorigenesis: context matters. Nat Rev Cancer 17, 239–253 (2017).
Eloubeidi, M. A. et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol 98, 2663–8 (2003).
Eloubeidi, M. A. et al. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine needle aspiration in suspected pancreatic cancer. J Gastrointest Surg 11, 813–9 (2007).
Giovannini, M. et al. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy 38, 344–8 (2006).
Chung, J. et al. The minimal amount of starting DNA for Agilent’s hybrid capture-based targeted massively parallel sequencing. Sci Rep 6, 26732 (2016).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–95 (2010).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–9 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20, 1297–303 (2010).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31, 213–9 (2013).
Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22, 568–76 (2012).
van Ginkel, J. H., Huibers, M. M. H., van Es, R. J. J., de Bree, R. & Willems, S. M. Droplet digital PCR for detection and quantification of circulating tumor DNA in plasma of head and neck cancer patients. BMC Cancer 17, 428 (2017).
Acknowledgements
We thank the technical staff of the Samsung Genome Institute for next generation sequencing. This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI13C2096), the National Research Foundation of Korea (NRF) Grants funded by the Korean Government (MSIT) (2017M3A9G5060264, 2017M3A9A7050803, and 2018M3C9A6017315), and a grant from Ministry of Food & Drug Safety, Republic of Korea (16173MFDS004).
Author information
Authors and Affiliations
Contributions
J.P., W.-Y.P., K.H.L. and D.P. conceived and organized this study; G.P., J.P., W.-Y.P., K.H.L. and D.P. designed the experimental framework; J.P. and K.H.L. collected samples and clinical data; G.P., Y.K., H.-J.J. and J.L. prepared samples and generated sequence data; G.P., D.-S.S. and S.-H.S. performed computational analysis; W.-Y.P. and D.P. supervised the experiments and the analysis; G.P., J.P., W.-Y.P. and D.P. wrote the manuscript; All authors contributed to and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, G., Park, J.K., Son, DS. et al. Utility of targeted deep sequencing for detecting circulating tumor DNA in pancreatic cancer patients. Sci Rep 8, 11631 (2018). https://doi.org/10.1038/s41598-018-30100-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30100-w
This article is cited by
-
High somatic mutations in circulating tumor DNA predict response of metastatic pancreatic ductal adenocarcinoma to first-line nab-paclitaxel plus S-1: prospective study
Journal of Translational Medicine (2024)
-
„Liquid biopsy“ in der gastrointestinalen Onkologie: Hype oder bald Realität?
Journal für Gastroenterologische und Hepatologische Erkrankungen (2022)
-
Characterization of DNA lesions associated with cell-free DNA by targeted deep sequencing
BMC Medical Genomics (2021)
-
Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer
Nature Reviews Gastroenterology & Hepatology (2020)
-
Impact of circulating tumor DNA in hepatocellular and pancreatic carcinomas
Journal of Cancer Research and Clinical Oncology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.