Abstract
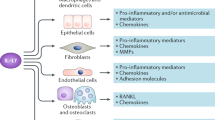
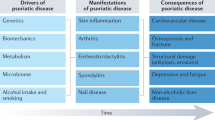
Over the past several years, a pathophysiological role for the IL-23–IL-17 pathway in human disease has been defined. A subset of rheumatic diseases, including psoriatic arthritis (PsA) and ankylosing spondylitis (AS), are now acknowledged to be triggered by dysregulated IL-23–IL-17 pathway activation. Genetic evidence links the IL-23–IL-17 pathway to inflammation in these rheumatic diseases, and mechanistic data from mice support a functional role for IL-23–IL-17 pathway activation in the development of enthesitis and in entheseal bone formation. Furthermore, analysis of human tissue samples, as well as data from clinical trials, also supports a role for activation of the IL-23–IL-17 pathway in these diseases. The unique bone phenotype that occurs in PsA and AS is a surprising coexistence of both systemic bone loss and periosteal and entheseal bone formation and is likely to be the result of the actions of IL-23 and/or IL-17 on bone. However, the effects of these cytokines on bone cells are complex, and controversy remains regarding their exact roles in the specific bone microenvironments relevant to PsA and AS.
Key points
-
IL-23 is produced by activated myeloid cells, whereas IL-17 is predominantly produced by T cells and innate lymphoid cells.
-
Several lines of evidence support a role for the IL-23–IL-17 pathway in the pathogenesis of psoriatic arthritis (PsA) and ankylosing spondylitis (AS).
-
Bone changes that occur in PsA and AS include systemic bone loss, articular erosions and entheseal bone formation and reflect the combined effects of IL-23 and IL-17.
-
IL-17A promotes osteoclastogenesis directly, as well as indirectly, through the production or induction of receptor-activator of nuclear factor-κB ligand (RANKL) expression, whereas the effects of IL-23 on osteoclasts are pleotropic.
-
IL-17A exhibits differential effects on the maturation of osteoblast precursor cells to osteoblasts depending upon the stage of differentiation of the cellular precursor.
-
IL-17A blockade inhibits articular bone erosion and might also retard systemic bone loss in PsA and AS and enthesophyte formation in PsA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Song, X., He, X., Li, X. & Qian, Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell. Mol. Immunol. 13, 418–431 (2016).
Boutet, M. A., Nerviani, A., Gallo Afflitto, G. & Pitzalis, C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int. J. Mol. Sci. 19, e530 (2018).
Ranganathan, V., Gracey, E., Brown, M. A., Inman, R. D. & Haroon, N. Pathogenesis of ankylosing spondylitis — recent advances and future directions. Nat. Rev. Rheumatol. 13, 359–367 (2017).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Oppmann, B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000).
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013).
Ghoreschi, K. et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Montaldo, E., Juelke, K. & Romagnani, C. Group 3 innate lymphoid cells (ILC3s): Origin, differentiation, and plasticity in humans and mice. Eur. J. Immunol. 45, 2127–2182 (2015).
Keijsers, R. R., Joosten, I., van Erp, P. E., Koenen, H. J. & van de Kerkhof, P. C. Cellular sources of IL-17 in psoriasis: a paradigm shift? Exp. Dermatol. 23, 799–803 (2014).
Razawy, W., van Driel, M. & Lubberts, E. The role of IL-23 receptor signaling in inflammation-mediated erosive autoimmune arthritis and bone remodeling. Eur. J. Immunol. 48, 220–229 (2018).
Huffmeier, U. et al. Genetic variants of the IL-23R pathway: association with psoriatic arthritis and psoriasis vulgaris, but no specific risk factor for arthritis. J. Invest. Dermatol. 129, 355–358 (2009).
Wellcome Trust Case Control Consortium et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Farh, K. K. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343 (2015).
Uddin, M. et al. Integrated genomics identifies convergence of ankylosing spondylitis with global immune mediated disease pathways. Sci. Rep. 5, 10314 (2015).
Sarin, R., Wu, X. & Abraham, C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T cell functional responses. Proc. Natl Acad. Sci. USA 108, 9560–9565 (2011).
Di Meglio, P. et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS ONE 6, e17160 (2011).
van Duivenvoorde, L. M. et al. Relationship between inflammation, bone destruction, and osteoproliferation in the HLA-B27/human β2 -microglobulin-transgenic rat model of spondylarthritis. Arthritis Rheum. 64, 3210–3219 (2012).
Aschermann, S. et al. Presence of HLA-B27 is associated with changes of serum levels of mediators of the Wnt and hedgehog pathway. Joint Bone Spine 83, 43–46 (2016).
Appel, H. et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 60, 3257–3262 (2009).
Neerinckx, B., Kollnberger, S., Shaw, J. & Lories, R. No evidence for a direct role of HLA-B27 in pathological bone formation in axial SpA. RMD Open 3, e000451 (2017).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Utriainen, L. et al. Expression of HLA-B27 causes loss of migratory dendritic cells in a rat model of spondyloarthritis. Arthritis Rheum. 64, 3199–3209 (2012).
Ebihara, S., Date, F., Dong, Y. & Ono, M. Interleukin-17 is a critical target for the treatment of ankylosing enthesitis and psoriasis-like dermatitis in mice. Autoimmunity 48, 259–266 (2015).
Abe, Y. et al. Ankylosing enthesitis associated with up-regulated IFN-γ and IL-17 production in (BXSB×NZB) F(1) male mice: a new mouse model. Mod. Rheumatol. 19, 316–322 (2009).
Shen, H., Goodall, J. C. & Hill Gaston, J. S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 (2009).
Zhang, L. et al. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS ONE 7, e31000 (2012).
Kenna, T. J. et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 64, 1420–1429 (2012).
Mei, Y. et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin. Rheumatol. 30, 269–273 (2011).
Celis, R. et al. Synovial cytokine expression in psoriatic arthritis and associations with lymphoid neogenesis and clinical features. Arthritis Res. Ther. 14, R93 (2012).
Appel, H. et al. Analysis of IL-17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 13, R95 (2011).
Cuthbert, R. J. et al. Brief report: group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol. 69, 1816–1822 (2017).
Baeten, D. et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382, 1705–1713 (2013).
Mease, P. J. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 373, 1329–1339 (2015).
Mease, P. J. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 76, 79–87 (2017).
Ritchlin, C. et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 73, 990–999 (2014).
McInnes, I. B. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382, 780–789 (2013).
Araujo, E. G. et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin. Arthritis Rheum. https://doi.org/10.1016/j.semarthrit.2018.05.011 (2018).
Baeten, D. et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 77, 1295–1302 (2018).
Poddubnyy, D., Hermann, K. G., Callhoff, J., Listing, J. & Sieper, J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 73, 817–823 (2014).
Walsh, N. C. et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J. Bone Miner. Res. 24, 1572–1585 (2009).
Kocijan, R. et al. Quantitative and qualitative changes of bone in psoriasis and psoriatic arthritis patients. J. Bone Miner. Res. 30, 1775–1783 (2015).
Devogelaer, J. P., Maldague, B., Malghem, J. & Nagant de Deuxchaisnes, C. Appendicular and vertebral bone mass in ankylosing spondylitis. A comparison of plain radiographs with single- and dual-photon absorptiometry and with quantitative computed tomography. Arthritis Rheum. 35, 1062–1067 (1992).
Donnelly, S. et al. Bone mineral density and vertebral compression fracture rates in ankylosing spondylitis. Ann. Rheum. Dis. 53, 117–121 (1994).
Ogdie, A. et al. The risk of fracture among patients with psoriatic arthritis and psoriasis: a population-based study. Ann. Rheum. Dis. 76, 882–885 (2017).
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802 (2012).
Kleyer, A. et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann. Rheum. Dis. 73, 854–860 (2014).
Neerinckx, B. & Lories, R. Mechanisms, impact and prevention of pathological bone regeneration in spondyloarthritis. Curr. Opin. Rheumatol. 29, 287–292 (2017).
Simon, D. et al. Analysis of periarticular bone changes in patients with cutaneous psoriasis without associated psoriatic arthritis. Ann. Rheum. Dis. 75, 660–666 (2016).
Takayanagi, H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 (2007).
Schett, G. et al. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch. Intern. Med. 166, 2495–2501 (2006).
Gravallese, E. M. et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 43, 250–258 (2000).
Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Schett, G. Review: immune cells and mediators of inflammatory arthritis. Autoimmunity 41, 224–229 (2008).
Walsh, N. C. & Gravallese, E. M. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr. Opin. Rheumatol. 16, 419–427 (2004).
Danks, L. et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann. Rheum. Dis. 75, 1187–1195 (2016).
Cohen, S. B. et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 58, 1299–1309 (2008).
Takeuchi, T. et al. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with rheumatoId arthritis on methotrexate to validate inhibitory effect on bone erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann. Rheum. Dis. 75, 983–990 (2016).
Daiichi Sankyo Company. Daiichi Sankyo obtains approval for additional indication for PRALIA® subcutaneous injection 60 mg syringe. Daiichi-Sankyo https://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/006655.html (2017).
Takayanagi, H. et al. T cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408, 600–605 (2000).
Saleh, H. et al. Interleukin-33, a target of parathyroid hormone and oncostatin M, increases osteoblastic matrix mineral deposition and inhibits osteoclast formation in vitro. Endocrinology 152, 1911–1922 (2011).
Kotake, S. et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352 (1999).
Kim, K. W., Kim, H. R., Kim, B. M., Cho, M. L. & Lee, S. H. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am. J. Pathol. 185, 3011–3024 (2015).
Lee, Y. The role of interleukin-17 in bone metabolism and inflammatory skeletal diseases. BMB Rep. 46, 479–483 (2013).
Sato, K. et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203, 2673–2682 (2006).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Pollinger, B. et al. Th17 cells, not IL-17+ γδT cells, drive arthritic bone destruction in mice and humans. J. Immunol. 186, 2602–2612 (2011).
Yago, T. et al. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-α antibody: a novel mechanism of osteoclastogenesis by IL-17. J. Cell. Biochem. 108, 947–955 (2009).
Adamopoulos, I. E. et al. Interleukin-17A upregulates receptor activator of NF-κB on osteoclast precursors. Arthritis Res. Ther. 12, R29 (2010).
Katz, Y., Nadiv, O. & Beer, Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 44, 2176–2184 (2001).
Jovanovic, D. V. et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 160, 3513–3521 (1998).
van der Heijde, D. et al. Brief report: Secukinumab provides significant and sustained inhibition of joint structural damage in a phase III study of active psoriatic arthritis. Arthritis Rheumatol. 68, 1914–1921 (2016).
Huang, H. et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 16, 1332–1343 (2009).
Osta, B., Lavocat, F., Eljaafari, A. & Miossec, P. Effects of interleukin-17A on osteogenic differentiation of isolated human mesenchymal stem cells. Front. Immunol. 5, 425 (2014).
Goswami, J., Hernandez-Santos, N., Zuniga, L. A. & Gaffen, S. L. A bone-protective role for IL-17 receptor signaling in ovariectomy-induced bone loss. Eur. J. Immunol. 39, 2831–2839 (2009).
DeSelm, C. J. et al. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. J. Cell. Biochem. 113, 2895–2902 (2012).
Uluckan, O. et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci. Transl Med. 8, 330ra37 (2016).
Shaw, A. T., Maeda, Y. & Gravallese, E. M. IL-17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis. Arthritis Res. Ther. 18, 104–113 (2016).
Kim, Y. G. et al. IL-17 inhibits osteoblast differentiation and bone regeneration in rat. Arch. Oral Biol. 59, 897–905 (2014).
Ono, T. et al. IL-17-producing γδ T cells enhance bone regeneration. Nat. Commun. 7, 10928 (2016).
Nam, D. et al. T-Lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PLoS ONE 7, e40044 (2012).
Croes, M. et al. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 84, 262–270 (2016).
Yago, T. et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res. Ther. 9, R96 (2007).
Ju, J. H. et al. IL-23 induces receptor activator of NF-κB ligand expression on CD4+ T cells and promotes osteoclastogenesis in an autoimmune arthritis model. J. Immunol. 181, 1507–1518 (2008).
Li, X. et al. IL-23 induces receptor activator of NF-κB ligand expression in fibroblast-like synoviocytes via STAT3 and NF-κB signal pathways. Immunol. Lett. 127, 100–107 (2010).
Chen, L., Wei, X. Q., Evans, B., Jiang, W. & Aeschlimann, D. IL-23 promotes osteoclast formation by up-regulation of receptor activator of NF-κB (RANK) expression in myeloid precursor cells. Eur. J. Immunol. 38, 2845–2854 (2008).
Shin, H. S. et al. Crosstalk among IL-23 and DNAX activating protein of 12 kDa-dependent pathways promotes osteoclastogenesis. J. Immunol. 194, 316–324 (2015).
Kamiya, S. et al. Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation. J. Bone Miner. Metab. 25, 277–285 (2007).
Quinn, J. M. et al. IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J. Immunol. 181, 5720–5729 (2008).
Adamopoulos, I. E. et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J. Immunol. 187, 951–959 (2011).
Kavanaugh, A. et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann. Rheum. Dis. 73, 1000–1006 (2014).
Pfeifle, R. et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 18, 104–113 (2017).
Harre, U. et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat. Commun. 6, 6651 (2015).
Zhang, J. R. et al. Different modulatory effects of IL-17, IL-22, and IL-23 on osteoblast differentiation. Mediators Inflamm. 2017, 5950395 (2017).
Reinhardt, A. & Prinz, I. Whodunit? The contribution of interleukin (IL)-17/IL-22- producing γδ T cells, αβ cells T cells, and innate lymphoid cells to the pathogenesis of spondyloarthritis. Front. Immunol. 9, 885 (2018).
Karczewski, J., Dobrowolska, A., Rychlewska-Hanczewska, A. & Adamski, Z. New insights into the role of T cells in pathogenesis of psoriasis and psoriatic arthritis. Autoimmunity 49, 435–450 (2016).
Mitra, A., Raychaudhuri, S. K. & Raychaudhuri, S. P. Functional role of IL-22 in psoriatic arthritis. Arthritis Res. Ther. 14, R65 (2012).
Yang, L. et al. Augmented Th17 differentiation leads to cutaneous and synovio-entheseal inflammation in a novel model of psoriatic arthritis. Arthritis Rheumatol. 70, 855–867 (2018).
Kim, K. W. et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 64, 1015–1023 (2012).
El-Zayadi, A. A. et al. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology 56, 488–493 (2017).
McGonagle, D., Lories, R. J., Tan, A. L. & Benjamin, M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 56, 2482–2491 (2007).
Lisowska, B., Kosson, D. & Domaracka, K. Lights and shadows of NSAIDs in bone healing: the role of prostaglandins in bone metabolism. Drug Des. Devel. Ther. 12, 1753–1758 (2018).
Wang, Z. et al. The positive effects of secreting cytokines IL-17 and IFN-γ on the early-stage differentiation and negative effects on the calcification of primary osteoblasts in vitro. Int. Immunopharmacol. 57, 1–10 (2018).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548 (2015).
Braun, J. et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann. Rheum. Dis. 76, 1070–1077 (2017).
Landewe, R., Dougados, M., Mielants, H., van der Tempel, H. & van der Heijde, D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann. Rheum. Dis. 68, 863–867 (2009).
Kampylafka, E. Resolution of synovitis and arrest of catabolic and anabolic bone changes in patients with psoriaric arthritis by IL-17 blockade with secukinumab; results from the prospective PSARTROS study. Arthritis Res. Ther. 20, 153 (2018).
Acknowledgements
The authors thank S. Williams for editorial assistance. The work of E.M.G. was partially supported by the Timothy and Elaine Peterson Research Fund (UMMS; P60037138450000). The work of G.S. was partially supported by the German Research Council (DFG; CRC1181).
Reviewer information
Nature Reviews Rheumatology thanks A. Deodhar, E. Lubberts and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
E.M.G. declares that she has received research funding from AbbVie and Eli Lilly. G.S. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gravallese, E.M., Schett, G. Effects of the IL-23–IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol 14, 631–640 (2018). https://doi.org/10.1038/s41584-018-0091-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-018-0091-8
This article is cited by
-
PriPath: identifying dysregulated pathways from differential gene expression via grouping, scoring, and modeling with an embedded feature selection approach
BMC Bioinformatics (2023)
-
Preosteoclast plays a pathogenic role in syndesmophyte formation of ankylosing spondylitis through the secreted PDGFB — GRB2/ERK/RUNX2 pathway
Arthritis Research & Therapy (2023)
-
Serum IL-23 significantly decreased in obese patients with psoriatic arthritis six months after a structured weight loss intervention
Arthritis Research & Therapy (2023)
-
Spondyloarthritis with inflammatory bowel disease: the latest on biologic and targeted therapies
Nature Reviews Rheumatology (2023)
-
Elevated serum IL-36γ levels in patients with ankylosing spondylitis and its association with disease activity
Molecular and Cellular Biochemistry (2023)