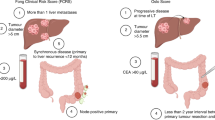
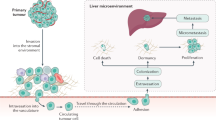
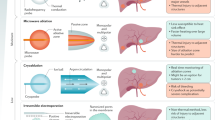
Abstract
Malignant liver tumours include a wide range of primary and secondary tumours. Although surgery remains the mainstay of curative treatment, modern therapies integrate a variety of neoadjuvant and adjuvant strategies and have achieved dramatic improvements in survival. Extensive tumour loads, which have traditionally been considered unresectable, are now amenable to curative treatment through systemic conversion chemotherapies followed by a variety of interventions such as augmentation of the healthy liver through portal vein occlusion, staged surgeries or ablation modalities. Liver transplantation is established in selected patients with hepatocellular carcinoma but is now emerging as a promising option in many other types of tumour such as perihilar cholangiocarcinomas, neuroendocrine or colorectal liver metastases. In this Review, we summarize the available therapies for the treatment of malignant liver tumours, with an emphasis on surgical and ablative approaches and how they align with other therapies such as modern anticancer drugs or radiotherapy. In addition, we describe three complex case studies of patients with malignant liver tumours. Finally, we discuss the outlook for future treatment, including personalized approaches based on molecular tumour subtyping, response to targeted drugs, novel biomarkers and precision surgery adapted to the specific tumour.
Key points
-
Contraindications for two-stage hepatectomy owing to a small future liver remnant should always be evaluated at expert centres.
-
The minimally invasive resection of selective liver tumours is oncologically efficient and offers faster postoperative recovery than invasive procedures.
-
Ablation therapy is an essential component of the treatment of liver tumours as it enables parenchymal preservation and treatment of high-risk patients and can be applied in combination with liver resection or as a bridge to liver transplantation.
-
Liver transplantation and transplant oncology are evolving as life-saving treatment options for patients with otherwise unresectable liver tumours.
-
Stereotactic body radiotherapy is a non-invasive treatment that achieves local tumour control in challenging situations such as when tumour size is >3 cm and tumour location is close to central vessels and the biliary system.
-
Systemic therapy is used to convert unresectable tumours into resectable tumours, target micrometastatic disease and provide disease control for staged procedures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Clavien, P. A., Petrowsky, H., DeOliveira, M. L. & Graf, R. Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 356, 1545–1559 (2007). This review indicates the significance of safer strategies in liver surgery and partial liver transplantation and highlights tissue augmentation approaches by portal vein embolization and two-stage hepatectomy.
Cabibbo, G., Latteri, F., Antonucci, M. & Craxi, A. Multimodal approaches to the treatment of hepatocellular carcinoma. Nat. Clin. Pract. Gastroenterol. Hepatol. 6, 159–169 (2009).
Cassidy, S. & Syed, B. A. Colorectal cancer drugs market. Nat. Rev. Drug Discov. 16, 525–526 (2017).
Adam, R. et al. 2018 annual report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl. Int. 31, 1293–1317 (2018).
Bismuth, H. et al. Hepatic transplantation in Europe. First report of the European Liver Transplant Registry. Lancet 2, 674–676 (1987).
Sapisochin, G., et al. Transplant oncology in primary and metastatic liver tumor: principles, evidence and opportunities. Ann. Surg. https://doi.org/10.1097/SLA.0000000000004071 (2020).
Ignatavicius, P. et al. Choices of therapeutic strategies for colorectal liver metastases among expert liver surgeons. A throw of the dice? Ann. Surg. https://doi.org/10.1097/SLA.0000000000004331 (2020).
Jarnagin, W. R. et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann. Surg. 236, 397–406 (2002).
Poon, R. T. et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann. Surg. 240, 698–708 (2004).
Smyrniotis, V., Kostopanagiotou, G., Theodoraki, K., Tsantoulas, D. & Contis, J. C. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am. J. Surg. 187, 398–402 (2004).
Pringle, J. H. V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann. Surg. 48, 541–549 (1908).
Lesurtel, M., Selzner, M., Petrowsky, H., McCormack, L. & Clavien, P. A. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann. Surg. 242, 814–822 (2005).
Couinaud, C. Liver lobes and segments: notes on the anatomical architecture and surgery of the liver [In French]. Presse Med. 62, 709–712 (1954).
Frilling, A. & Clift, A. K. Surgical approaches to the management of neuroendocrine liver metastases. Endocrinol. Metab. Clin. North Am. 47, 627–643 (2018).
Sano, K. et al. Outcomes of 1,639 hepatectomies for non-colorectal non-neuroendocrine liver metastases: a multicenter analysis. J. Hepatobiliary Pancreat. Sci. 25, 465–475 (2018).
Lai, Q. et al. Recurrence of hepatocellular cancer after liver transplantation: the role of primary resection and salvage transplantation in East and West. J. Hepatol. 57, 974–979 (2012).
He, S. et al. Effect of prophylactic TACE on 5-year survival of patients with hepatocellular carcinoma after hepatectomy. Oncol. Lett. 18, 1824–1830 (2019).
Ogata, S. et al. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br. J. Surg. 93, 1091–1098 (2006).
Shim, J. H. et al. Complete necrosis after transarterial chemoembolization could predict prolonged survival in patients with recurrent intrahepatic hepatocellular carcinoma after curative resection. Ann. Surg. Oncol. 17, 869–877 (2010).
Vigano, L. et al. Minor hepatectomies: focusing a blurred picture: analysis of the outcome of 4471 open resections in patients without cirrhosis. Ann. Surg. 270, 842–851 (2019).
Mise, Y. et al. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann. Surg. 263, 146–152 (2016).
Kobayashi, K. et al. Parenchyma-sparing liver resection for hepatocellular carcinoma in left lateral section is associated with better liver volume recovery. HPB 20, 949–955 (2018).
Torzilli, G. et al. Hepatic vein management in a parenchyma-sparing policy for resecting colorectal liver metastases at the caval confluence. Surgery 163, 277–284 (2018).
Famularo, S. et al. Long-term oncologic results of anatomic vs. parenchyma-sparing resection for hepatocellular carcinoma. A propensity score-matching analysis. Eur. J. Surg. Oncol. 44, 1580–1587 (2018).
Moris, D. et al. Parenchymal-sparing versus anatomic liver resection for colorectal liver metastases: a systematic review. J. Gastrointest. Surg. 21, 1076–1085 (2017).
Lordan, J. T. et al. Case-controlled study comparing peri-operative and cancer-related outcomes after major hepatectomy and parenchymal sparing hepatectomy for metastatic colorectal cancer. HPB 19, 688–694 (2017).
Kalil, J. A. et al. Laparoscopic parenchymal-sparing hepatectomy: the new maximally minimal invasive surgery of the liver-a systematic review and meta-analysis. J. Gastrointest. Surg. 23, 860–869 (2019).
Rous, P. & Larimore, L. D. Relation of the portal blood to liver maintenance: a demonstration of liver atrophy conditional on compensation. J. Exp. Med. 31, 609–632 (1920).
Makuuchi, M. et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 107, 521–527 (1990).
Adam, R., Laurent, A., Azoulay, D., Castaing, D. & Bismuth, H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann. Surg. 232, 777–785 (2000).
Jaeck, D. et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann. Surg. 240, 1037–1049 (2004). This article first reported a two-stage hepatectomy strategy combined with portal vein embolization to convert unresectable scenarios into resectable scenarios.
Kianmanesh, R. et al. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J. Am. Coll. Surg. 197, 164–170 (2003).
Kawaguchi, Y., Lillemoe, H. A. & Vauthey, J. N. Dealing with an insufficient future liver remnant: portal vein embolization and two-stage hepatectomy. J. Surg. Oncol. 119, 594–603 (2019).
Elias, D. et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br. J. Surg. 86, 784–788 (1999).
Heinrich, S., Jochum, W., Graf, R. & Clavien, P. A. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and liver regeneration in mice. J. Hepatol. 45, 35–42 (2006).
Esposito, F. et al. Combined hepatic and portal vein embolization as preparation for major hepatectomy: a systematic review. HPB 21, 1099–1106 (2019).
Chiche, L. et al. Radiological simultaneous porto-hepatic vein embolization (RASPE) before major hepatectomy: a better way to optimize liver hypertrophy compared to portal vein embolization. Ann. Surg. https://doi.org/10.1097/SLA.0000000000003905 (2020).
Clavien, P. A. Hepatic vein embolization for safer liver surgery insignificant novelty or a breakthrough? Ann. Surg. https://doi.org/10.1097/SLA.0000000000003973 (2020).
Schnitzbauer, A. A. et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 255, 405–414 (2012). This article reports, for the first time, that additional in situ splitting results in rapid and efficient hypertrophy in a two-stage hepatectomy approach.
Lang, H. et al. 10th anniversary of ALPPS-lessons learned and quo vadis. Ann. Surg. 269, 114–119 (2019).
de Santibanes, E. & Clavien, P. A. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann. Surg. 255, 415–417 (2012).
Linecker, M. et al. Risk adjustment in ALPPS is associated with a dramatic decrease in early mortality and morbidity. Ann. Surg. 266, 779–786 (2017).
Wang, Z. et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann. Surg. 271, 534–541 (2020).
Linecker, M. et al. ALPPS in neuroendocrine liver metastases not amenable for conventional resection - lessons learned from an interim analysis of the International ALPPS Registry. HPB 22, 537–544 (2020).
Li, J. et al. ALPPS for locally advanced intrahepatic cholangiocarcinoma: did aggressive surgery lead to the oncological benefit? An international multi-center study. Ann. Surg. Oncol. 27, 1372–1384 (2020).
Truant, S. et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur. J. Surg. Oncol. 41, 674–682 (2015).
Kremer, M. et al. Impact of neoadjuvant chemotherapy on hypertrophy of the future liver remnant after associating liver partition and portal vein ligation for staged hepatectomy. J. Am. Coll. Surg. 221, 717–728.e1 (2015).
Schadde, E. et al. Early survival and safety of ALPPS: first report of the international ALPPS registry. Ann. Surg. 260, 829–836 (2014).
Raptis, D. A. et al. Defining Benchmark Outcomes for ALPPS. Ann. Surg. 270, 835–841 (2019).
Eshmuminov, D. et al. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br. J. Surg. 103, 1768–1782 (2016).
Sandstrom, P. et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a scandinavian multicenter randomized controlled trial (LIGRO Trial). Ann. Surg. 267, 833–840 (2018).
Hasselgren, K., Røsok, B. I., Sparrelid, E., Sandström, P. ALPPS improves survival compared with TSH in patients affected of CRLM — survival analysis from the randomized controlled trial LIGRO. Ann. Surg. https://doi.org/10.1097/SLA.0000000000003701 (2019). This randomized trial demonstrates in patients with CRLM significantly superior survival after ALPPS than after two-stage hepatectomy.
Li, J. et al. Avoid “All-Touch” by hybrid ALPPS to achieve oncological efficacy. Ann. Surg. 263, e6–e7 (2016).
Linecker, M. et al. How much liver needs to be transected in ALPPS? A translational study investigating the concept of less invasiveness. Surgery 161, 453–464 (2017).
Petrowsky, H., Gyori, G., de Oliveira, M., Lesurtel, M. & Clavien, P. A. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann. Surg. 261, e90–e92 (2015).
Machado, M. A., Makdissi, F. F. & Surjan, R. C. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann. Surg. 256, e13 (2012).
Robles, R. et al. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br. J. Surg. 101, 1129–1134 (2014).
de Santibanes, E., Alvarez, F. A., Ardiles, V., Pekolj, J. & de Santibanes, M. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini-ALPPS technique. Langenbecks Arch. Surg. 401, 557–563 (2016).
Rosok, B. I. et al. Characterization of early recurrences following liver resection by ALPPS and two stage hepatectomy in patients with colorectal liver-metastases and small future liver remnants; a translational substudy of the LIGRO-RCT. HPB 21, 1017–1023 (2019).
Linecker, M., Kuemmerli, C., Clavien, P. A. & Petrowsky, H. Dealing with insufficient liver remnant: associating liver partition and portal vein ligation for staged hepatectomy. J. Surg. Oncol. 119, 604–612 (2019).
Mentha, G. et al. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br. J. Surg. 93, 872–878 (2006).
Clavien, P. A. & Barkun, J. Consensus conference on laparoscopic liver resection: a jury-based evaluation. Ann. Surg. 261, 630–631 (2015).
Wakabayashi, G. et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann. Surg. 261, 619–629 (2015). International Consensus Conference on Laparoscopic Liver Resections to evaluate the current status of laparoscopic liver surgery and to provide recommendations to aid its future development.
Abu Hilal, M. et al. The southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann. Surg. 268, 11–18 (2018).
Fruscione, M. et al. Robotic-assisted versus laparoscopic major liver resection: analysis of outcomes from a single center. HPB 21, 906–911 (2019).
Fahrner, R. et al. Robotic hepatic surgery in malignancy: review of the current literature. J. Robot. Surg. 13, 533–538 (2019).
Kim, J. K. et al. Robotic versus laparoscopic left lateral sectionectomy of liver. Surg. Endosc. 30, 4756–4764 (2016).
Salloum, C. et al. Robotic-assisted versus laparoscopic left lateral sectionectomy: analysis of surgical outcomes and costs by a propensity score matched cohort study. World J. Surg. 41, 516–524 (2017).
Chen, P. D. et al. Robotic versus open hepatectomy for hepatocellular carcinoma: a matched comparison. Ann. Surg. Oncol. 24, 1021–1028 (2017).
Chen, P. D. et al. Robotic major hepatectomy: is there a learning curve? Surgery 161, 642–649 (2017).
Barkun, J. S., Dimick, J. B. & Clavien, P. A. Surgical research in patients: ideal time for an IDEAL checklist. Ann. Surg. 269, 208–210 (2019).
Hirst, A. et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann. Surg. 269, 211–220 (2019).
Halls, M. C. et al. A comparison of the learning curves of laparoscopic liver surgeons in differing stages of the IDEAL paradigm of surgical innovation: standing on the shoulders of pioneers. Ann. Surg. 269, 221–228 (2019).
Rogers, W. A., Hutchison, K. & McNair, A. Ethical issues across the IDEAL stages of surgical innovation. Ann. Surg. 269, 229–233 (2019).
Cortolillo, N., Patel, C., Parreco, J., Kaza, S. & Castillo, A. Nationwide outcomes and costs of laparoscopic and robotic vs. open hepatectomy. J. Robot. Surg. 13, 557–565 (2019).
Cillo, U. et al. Videolaparoscopic microwave ablation in patients with HCC at a European high-volume center: results of 815 procedures. J. Surg. Oncol. 120, 956–965 (2019).
Cornelis, F. H. & Solomon, S. B. Treatment of primary liver tumors and liver metastases, part 2: non-nuclear medicine techniques. J. Nucl. Med. 59, 1801–1808 (2018).
Giglio, M. C. et al. Laparoscopic versus open thermal ablation of colorectal liver metastases: a propensity score-based analysis of local control of the ablated tumors. Ann. Surg. Oncol. https://doi.org/10.1245/s10434-020-08243-w (2020).
Ringe, K. I. et al. Experimental evaluation of the heat sink effect in hepatic microwave ablation. PLoS One 10, e0134301 (2015).
Schramm, W., Yang, D., Wood, B. J., Rattay, F. & Haemmerich, D. Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Open Biomed. Eng. J. 1, 47–52 (2007).
Ahmed, M., Brace, C. L., Lee, F. T. Jr & Goldberg, S. N. Principles of and advances in percutaneous ablation. Radiology 258, 351–369 (2011).
Ding, J. et al. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin. Radiol. 68, 608–615 (2013).
Seror, O. Ablative therapies: advantages and disadvantages of radiofrequency, cryotherapy, microwave and electroporation methods, or how to choose the right method for an individual patient? Diagn. Interv. Imaging 96, 617–624 (2015). This article points out the advantages of each ablation technique, highlighting important differences in their technologies and how to transfer this concept into clinical practice.
Adam, R. et al. Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann. Surg. 253, 349–359 (2011).
Loveman, E. et al. The clinical effectiveness and cost-effectiveness of ablative therapies in the management of liver metastases: systematic review and economic evaluation. Health Technol. Assess. 18, 1–283 (2014).
Faitot, F. et al. Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases: a case-matched analysis of surgical and oncological outcomes. Ann. Surg. 260, 822–827 (2014). This study compares the long-term follow-up of two surgical strategies for patients with bilobar CRLM. It demonstrates that the one-stage strategy combining resection with radiofrequency ablation is a good option in the treatment of such patients.
Park, H. M. et al. Outcomes for patients with recurrent intrahepatic cholangiocarcinoma after surgery. Ann. Surg. Oncol. 23, 4392–4400 (2016).
Yin, X. Y. et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer 115, 1914–1923 (2009).
Lee, M. W. et al. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a 10-year intention-to-treat analysis. Hepatology 65, 1979–1990 (2017).
Kim, Y. S. et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J. Hepatol. 58, 89–97 (2013).
Feng, K. et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 57, 794–802 (2012).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
Davalos, R. V., Mir, I. L. & Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 33, 223–231 (2005).
Dollinger, M. et al. Bile duct injury after irreversible electroporation of hepatic malignancies: evaluation of MR imaging findings and laboratory values. J. Vasc. Interv. Radiol. 27, 96–103 (2016).
Kambakamba, P. et al. Intraoperative adverse events during irreversible electroporation-a call for caution. Am. J. Surg. 212, 715–721 (2016).
Scheffer, H. J. et al. Colorectal liver metastatic disease: efficacy of irreversible electroporation–a single-arm phase II clinical trial (COLDFIRE-2 trial). BMC Cancer 15, 772 (2015).
Cannon, R., Ellis, S., Hayes, D., Narayanan, G. & Martin, R. C. II. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J. Surg. Oncol. 107, 544–549 (2013).
Cannon, R. M., Bolus, D. N. & White, J. A. Irreversible electroporation as a bridge to liver transplantation. Am. Surg. 85, 103–110 (2019).
Cheng, R. G., Bhattacharya, R., Yeh, M. M. & Padia, S. A. Irreversible electroporation can effectively ablate hepatocellular carcinoma to complete pathologic necrosis. J. Vasc. Interv. Radiol. 26, 1184–1188 (2015).
Mafeld, S. et al. Percutaneous irreversible electroporation (IRE) of hepatic malignancy: a bi-institutional analysis of safety and outcomes. Cardiovasc. Intervent. Radiol. 42, 577–583 (2019).
Coletti, L. et al. Safety and feasibility of electrochemotherapy in patients with unresectable colorectal liver metastases: a pilot study. Int. J. Surg. 44, 26–32 (2017).
Gasljevic, G. et al. Histopathological findings in colorectal liver metastases after electrochemotherapy. PLoS One 12, e0180709 (2017).
Funovics, J. M. et al. Primary hepatic cancer – the role of limited resection and total hepatectomy with orthotopic liver replacement. Hepatogastroenterology 35, 316–320 (1988).
Moris, D. et al. Liver transplantation for unresectable colorectal liver metastases: a systematic review. J. Surg. Oncol. 116, 288–297 (2017).
Rosenbrook, W. Jr & Carney, R. E. Spectinomycin modification. I 7-EPI-9-deoxy-4(R)-dihydrospectinomycin. J. Antibiot. 28, 953–959 (1975).
Bismuth, H., Majno, P. E. & Adam, R. Liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 19, 311–322 (1999).
Mazzaferro, V. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 334, 693–699 (1996).
Clavien, P. A. et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 13, e11–e22 (2012).
Wang, Z. Y., Geng, L. & Zheng, S. S. Current strategies for preventing the recurrence of hepatocellular carcinoma after liver transplantation. Hepatobiliary Pancreat. Dis. Int. 14, 145–149 (2015).
Sapisochin, G. & Bruix, J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat. Rev. Gastroenterol. Hepatol. 14, 203–217 (2017).
Yao, F. Y. et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33, 1394–1403 (2001).
Yao, F. Y. et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am. J. Transpl. 7, 2587–2596 (2007).
Yang, J. D. et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16, 589–604 (2019).
Muller, M., Bird, T. G. & Nault, J. C. The landscape of gene mutations in cirrhosis and hepatocellular carcinoma. J. Hepatol. 72, 990–1002 (2020).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71, 793–801 (2019).
Ma, X. L. et al. Significance of PIVKA-II levels for predicting microvascular invasion and tumor cell proliferation in Chinese patients with hepatitis B virus-associated hepatocellular carcinoma. Oncol. Lett. 15, 8396–8404 (2018).
Mazzaferro, V. et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 154, 128–139 (2018).
Cescon, M., Cucchetti, A., Ravaioli, M. & Pinna, A. D. Hepatocellular carcinoma locoregional therapies for patients in the waiting list. Impact on transplantability and recurrence rate. J. Hepatol. 58, 609–618 (2013).
Lesurtel, M., Mullhaupt, B., Pestalozzi, B. C., Pfammatter, T. & Clavien, P. A. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: an evidence-based analysis. Am. J. Transpl. 6, 2644–2650 (2006).
Hassoun, Z., Gores, G. J. & Rosen, C. B. Preliminary experience with liver transplantation in selected patients with unresectable hilar cholangiocarcinoma. Surg. Oncol. Clin. N. Am. 11, 909–921 (2002).
Rosen, C. B., Heimbach, J. K. & Gores, G. J. Liver transplantation for cholangiocarcinoma. Transpl. Int. 23, 692–697 (2010).
Darwish Murad, S. et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 143, 88–98.e3 (2012).
Ethun, C. G. et al. Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting treatment paradigms for resectable disease. Ann. Surg. 267, 797–805 (2018). The first intention-to-treat analysis in hilar cholangiocarcinoma to compare liver resection with liver transplantation in patients with the same tumour criteria (that is, <3 cm, lymph-node negative disease).
Resch, T. et al. Liver transplantation for hilar cholangiocarcinoma (h-CCA): is it the right time? Transl. Gastroenterol. Hepatol. 3, 38 (2018).
Sapisochin, G., Fernandez de Sevilla, E., Echeverri, J. & Charco, R. Liver transplantation for cholangiocarcinoma: current status and new insights. World J. Hepatol. 7, 2396–2403 (2015).
Sapisochin, G. et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology 64, 1178–1188 (2016).
Sapisochin, G. et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am. J. Transpl. 14, 660–667 (2014).
Lunsford, K. E. et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol. Hepatol. 3, 337–348 (2018).
Fujita, T. Liver transplantation for intrahepatic cholangiocarcinoma. Lancet 384, 1182 (2014).
Hagness, M. et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. 257, 800–806 (2013).
Line, P. D., Hagness, M., Berstad, A. E., Foss, A. & Dueland, S. A novel concept for partial liver transplantation in nonresectable colorectal liver metastases: the RAPID concept. Ann. Surg. 262, e5–e9 (2015). First report of RAPID, which combines regeneration of partial liver grafts followed by delayed resection of the remaining metastatic liver part.
Frilling, A. et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 15, e8–e21 (2014).
Lim, C. et al. Liver transplantation for neuroendocrine tumors: what have we learned? Semin. Liver Dis. 38, 351–356 (2018).
Richards-Taylor, S. et al. Clinically significant differences in Ki-67 proliferation index between primary and metastases in resected pancreatic neuroendocrine tumors. Pancreas 46, 1354–1358 (2017).
Dawson, L. A. et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int. J. Radiat. Oncol. Biol. Phys. 53, 810–821 (2002).
Wulf, J. et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 45, 838–847 (2006).
Guckenberger, M. et al. Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther. Onkol. 190, 26–33 (2014).
Lax, I., Blomgren, H., Naslund, I. & Svanstrom, R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 33, 677–683 (1994).
Chang, D. T. et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 117, 4060–4069 (2011).
Hoyer, M. et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 45, 823–830 (2006).
van der Pool, A. E. et al. Stereotactic body radiation therapy for colorectal liver metastases. Br. J. Surg. 97, 377–382 (2010).
Scorsetti, M. et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J. Cancer Res. Clin. Oncol. 141, 543–553 (2015).
Petrelli, F. et al. Stereotactic body radiotherapy for colorectal cancer liver metastases: a systematic review. Radiother. Oncol. 129, 427–434 (2018). Systematic review of SBRT for CRC liver metastases showing an association between SBRT dose and local metastases control.
Klement, R. J. et al. The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases. BMC Cancer 19, 173 (2019).
Toesca, D. A. et al. Central liver toxicity after SBRT: an expanded analysis and predictive nomogram. Radiother. Oncol. 122, 130–136 (2017).
Xi, M. et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One 8, e63864 (2013).
Van Cutsem, E. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
Kroeze, S. G. et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: a systematic review. Cancer Treat. Rev. 53, 25–37 (2017).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Vogel, A. et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30, 871–873 (2019).
Bujold, A. et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J. Clin. Oncol. 31, 1631–1639 (2013).
Takeda, A. et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 122, 2041–2049 (2016).
Bettinger, D. et al. Stereotactic body radiation therapy as an alternative treatment for patients with hepatocellular carcinoma compared to sorafenib: a propensity score analysis. Liver Cancer 8, 281–294 (2019).
Yoon, S. M. et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 4, 661–669 (2018). Randomized trial showing an overall survival benefit of TACE combined with SBRT compared to sorafenib in patients with advanced HCC showing macroscopic vascular invasion.
Uemura, T. et al. Stereotactic body radiation therapy: a new strategy for loco-regional treatment for hepatocellular carcinoma while awaiting liver transplantation. World J. Surg. 43, 886–893 (2019).
Sapisochin, G. et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J. Hepatol. 67, 92–99 (2017).
Wei, X. et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J. Clin. Oncol. 37, 2141–2151 (2019).
Mahadevan, A. et al. Stereotactic body radiotherapy (SBRT) for intrahepatic and hilar cholangiocarcinoma. J. Cancer 6, 1099–1104 (2015).
Gkika, E. et al. Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer 17, 781 (2017).
Feng, M. et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 4, 40–47 (2018).
Frakulli, R. et al. Stereotactic body radiation therapy in cholangiocarcinoma: a systematic review. Br. J. Radiol. 92, 20180688 (2019).
Mancini, R. et al. A multicentric phase II clinical trial on intra-arterial hepatic radiotherapy with 90yttrium SIR-spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy: preliminary results on toxicity and response rates. In Vivo 20, 711–714 (2006).
Bhooshan, N. et al. Pretreatment tumor volume as a prognostic factor in metastatic colorectal cancer treated with selective internal radiation to the liver using yttrium-90 resin microspheres. J. Gastrointest. Oncol. 7, 931–937 (2016).
Fendler, W. P. et al. Nomogram including pretherapeutic parameters for prediction of survival after SIRT of hepatic metastases from colorectal cancer. Eur. Radiol. 25, 2693–2700 (2015).
Wasan, H. S. et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 18, 1159–1171 (2017). Pooled analysis of three randomized trials showing that the addition of SIRT to first-line FOLFOX chemotherapy for patients with liver-only and liver-dominant mCRC did not improve overall survival compared with FOLFOX alone.
Chow, P. K. H. et al. SIRveNIB: selective internal radiation therapy versus sorafenib in asia-pacific patients with hepatocellular carcinoma. J. Clin. Oncol. 36, 1913–1921 (2018).
Vilgrain, V. et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 18, 1624–1636 (2017).
Braat, A. et al. Radioembolization with (90)Y resin microspheres of neuroendocrine liver metastases: international multicenter study on efficacy and toxicity. Cardiovasc. Intervent. Radiol. 42, 413–425 (2019).
Lehmann, K., Rickenbacher, A., Weber, A., Pestalozzi, B. C. & Clavien, P. A. Chemotherapy before liver resection of colorectal metastases: friend or foe? Ann. Surg. 255, 237–247 (2012).
Nordlinger, B. et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371, 1007–1016 (2008). The EPOC trial set the standard for perioperative treatment of technically resectable CRLM.
Primrose, J. et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the new EPOC randomised controlled trial. Lancet Oncol. 15, 601–611 (2014).
Valle, J. W. et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27 (Suppl. 5), v28–v37 (2016).
Primrose, J.N. et al. Adjuvant capecitabine for biliary tract cancer: The BILCAP randomized study. J. Clin. Oncol. 35, 4006–4006 (2017).
Stein, A. et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 15, 564 (2015).
Bruix, J. et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 16, 1344–1354 (2015).
Meyer, T. et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2, 565–575 (2017).
Ricke, J. et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 71, 1164–1174 (2019).
Zhu, X. D. & Sun, H. C. Emerging agents and regimens for hepatocellular carcinoma. J. Hematol. Oncol. 12, 110 (2019).
Duffy, A. G. et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 66, 545–551 (2017).
Greten, T. F., Mauda-Havakuk, M., Heinrich, B., Korangy, F. & Wood, B. J. Combined locoregional-immunotherapy for liver cancer. J. Hepatol. 70, 999–1007 (2019).
Pavel, M. et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 103, 172–185 (2016).
Kaltsas, G. et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: pre- and perioperative therapy in patients with neuroendocrine tumors. Neuroendocrinology 105, 245–254 (2017).
Strosberg, J. et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Partelli, S. et al. Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery 163, 761–767 (2018).
Adam, R. et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat. Rev. 41, 729–741 (2015).
Folprecht, G. et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 11, 38–47 (2010).
Adam, R. et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 17, 1225–1239 (2012).
Ye, L. C. et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J. Clin. Oncol. 31, 1931–1938 (2013).
Gruenberger, T. et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann. Oncol. 26, 702–708 (2015).
Geissler, M. et al. mFOLFOXIRI + panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer m(CRC): a randomized phase II VOLFI trial of the AIO (AIO- KRK0109). J. Clin. Oncol. 36, 3509–3509 (2018).
Cremolini, C. et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial JAMA Oncol. 4, 529–536 (2018).
Heinemann, V. et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur. J. Cancer 51, 1927–1936 (2015).
Shindoh, J. et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann. Surg. Oncol. 20, 2493–2500 (2013).
Chun, Y. S., Laurent, A., Maru, D. & Vauthey, J. N. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol. 10, 278–286 (2009).
Dienstmann, R., Salazar, R. & Tabernero, J. Molecular subtypes and the evolution of treatment decisions in metastatic colorectal cancer. Am. Soc. Clin. Oncol. Educ. Book 38, 231–238 (2018). This article provides an up-to-date overview of molecular subtyping and personalized systemic treatment of mCRC.
Arnold, D. et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 28, 1713–1729 (2017).
Pietrantonio, F. et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur. J. Cancer 51, 587–594 (2015).
Kopetz, S. et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N. Engl. J. Med. 381, 1632–1643 (2019).
Kopetz, S. et al. Updated results of the BEACON CRC safety lead-in: encorafenib (ENCO) + binimetinib (BINI) + cetuximab (CETUX) for BRAFV600E-mutant metastatic colorectal cancer (mCRC). J. Clin. Oncol. 37, 688–688 (2019).
Corcoran, R. B. et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 8, 428–443 (2018).
Raghav, R. et al. Validation of HER2 amplification as a predictive biomarker for anti–epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. JCO Precision Oncol. 3, 1–13 (2019).
Sartore-Bianchi, A. et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 738–746 (2016).
Le, D. et al. Safety and antitumor activity of pembrolizumab in patients with advanced microsatellite instability–high (MSI-H) colorectal cancer: KEYNOTE-164. Ann. Oncol. 29 (Suppl. 5), V107 (2018).
Lenz, H.-J. J. et al. Durable clinical benefit with nivolumab (NIVO) plus low-dose ipilimumab (IPI) as first-line therapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). Ann. Oncol. 29 (Suppl. 8), VIII714 (2018).
Le Roy, B. et al. Neoadjuvant chemotherapy initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 105, 839–847 (2018).
Jusakul, A. et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 7, 1116–1135 (2017).
Wainberg, Z. A. et al. Efficacy and safety of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated biliary tract cancer (BTC): a cohort of the ROAR basket trial. J. Clin. Oncol. 37, 187–187 (2019).
Abou-Alfa, G. K. ClarIDHy: a global, phase III, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutatio. Ann. Oncol. 30, v872–v873 (2019).
Vogel, A. FIGHT-202: A phase II study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA). Ann. Oncol. 30, v876 (2019).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Ikeda, M. et al. Abstract CT061: a phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Cancer Res. 79, CT061–CT061 (2019).
Llovet, J. M. et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): phase 3 LEAP-002 study. J. Clin. Oncol. 37, TPS4152 (2019).
Cheng, A.-L. LBA3 – IMbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann. Oncol. 30 (Suppl. 9), ix186–ix187 (2019).
Samaras, P. et al. Selective intra-arterial chemotherapy with floxuridine as second- or third-line approach in patients with unresectable colorectal liver metastases. Ann. Surg. Oncol. 18, 1924–1931 (2011).
Konstantinidis, I. T. et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 122, 758–765 (2016).
Cucchetti, A. et al. Selective internal radiation therapy (SIRT) as conversion therapy for unresectable primary liver malignancies. Liver Cancer 5, 303–311 (2016).
Fong, Y., Fortner, J., Sun, R. L., Brennan, M. F. & Blumgart, L. H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann. Surg. 230, 309–318 (1999).
Nordlinger, B. et al. Surgical resection of colorectal carcinoma metastases to the liver: A prognostic scoring system to improve case selection, based on 1568 patients. Cancer 77, 1254–1262 (1996).
Mann, C. D., Metcalfe, M. S., Leopardi, L. N. & Maddern, G. J. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch. Surg. 139, 1168–1172 (2004).
Balachandran, V. P. et al. A validated prognostic multigene expression assay for overall survival in resected colorectal cancer liver metastases. Clin. Cancer Res. 22, 2575–2582 (2016).
Pitroda, S. P. et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat. Commun. 9, 1793 (2018).
Mlecnik, B. et al. The tumor microenvironment and immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl Med. 8, 327ra326 (2016).
Tie, J. et al. Serial circulating tumor DNA (ctDNA) and recurrence risk in patients (pts) with resectable colorectal liver metastasis (CLM). J. Clin. Oncol. 34, e15131 (2016).
Acknowledgements
The authors thank S. Gaal for her dedication and very efficient work during the editorial process. The authors are supported by the Liver and Gastrointestinal Disease Foundation (LGID), Switzerland, and the Swiss National Foundation Grant ‘Division of tasks in the regenerating liver — metabolic restraints to successful recovery after tissue loss’.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Petrowsky, H., Fritsch, R., Guckenberger, M. et al. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol 17, 755–772 (2020). https://doi.org/10.1038/s41575-020-0314-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-0314-8
This article is cited by
-
Prognostic performance of MRI LI-RADS version 2018 features and clinical-pathological factors in alpha-fetoprotein-negative hepatocellular carcinoma
Abdominal Radiology (2024)
-
Robotic ALPPS for primary and metastatic liver tumours: short-term outcomes versus open approach
Updates in Surgery (2024)
-
Comprehensive analysis identifies CLEC1B as a potential prognostic biomarker in hepatocellular carcinoma
Cancer Cell International (2023)
-
EZH2 in hepatocellular carcinoma: progression, immunity, and potential targeting therapies
Experimental Hematology & Oncology (2023)
-
Application of the IDEAL framework in hepatopancreatobiliary surgery: a review of the literature
Langenbeck's Archives of Surgery (2023)