Abstract
Patients with localized pancreatic ductal adenocarcinoma (PDAC) are best treated with surgical resection of the primary tumour and systemic chemotherapy, which provides considerably longer overall survival (OS) durations than either modality alone. Regardless, most patients will have disease relapse owing to micrometastatic disease. Although currently a matter of some debate, considerable research interest has been focused on the role of neoadjuvant therapy for all forms of resectable PDAC. Whilst adjuvant combination chemotherapy remains the standard of care for patients with resectable PDAC, neoadjuvant chemotherapy seems to improve OS without necessarily increasing the resection rate in those with borderline-resectable disease. Furthermore, around 20% of patients with unresectable non-metastatic PDAC might undergo resection following 4–6 months of induction combination chemotherapy with or without radiotherapy, even in the absence of a clear radiological response, leading to improved OS outcomes in this group. Distinct molecular and biological responses to different types of therapies need to be better understood in order to enable the optimal sequencing of specific treatment modalities to further improve OS. In this Review, we describe current treatment strategies for the various clinical stages of PDAC and discuss developments that are likely to determine the optimal sequence of multimodality therapies by integrating the fundamental clinical and molecular features of the cancer.
Key points
-
Surgical resection of the primary tumour and adjuvant systemic combination chemotherapy is the most effective treatment for pancreatic cancer.
-
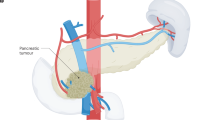
Three surgical stages have emerged for the preoperative assessment of non-metastatic pancreatic cancers: resectable, borderline-resectable and unresectable.
-
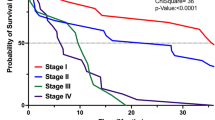
In patients with resectable pancreatic cancer who are able to receive adjuvant chemotherapy (usually for 6 months), 5-year overall survival (OS) of 30–50% can be expected.
-
In patients with borderline-resectable disease, the addition of a short course of neoadjuvant therapy (usually for 2 months) prior to surgery (with R0/R1 resection rates of 64–85%) increases 12-month OS to around 77%, compared with 40% for upfront surgery (with a resection rate of 75%); a longer course of neoadjuvant chemotherapy (usually for 4 months) can lead to an 18-month OS of 67%.
-
Selected patients with initially locally advanced, unresectable non-metastatic pancreatic cancer might become eligible for resection following 4–6 months of induction chemotherapy, with or without radiotherapy (with macroscopic resection rates as high as 20–40%), with improved OS compared with those who do not undergo resection.
-
The development of new neoadjuvant and induction strategies needs to integrate the distinct molecular and biological characteristics of the various pancreatic cancer subgroups to optimize the selection and sequencing of both established and novel treatment modalities in order to improve survival outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Park, W., Chawla, A. & O’Reilly, E. M. Pancreatic cancer: a review. JAMA 326, 851–862 (2021).
Klaiber, U., Hackert, T. & Neoptolemos, J. P. Adjuvant treatment for pancreatic cancer. Transl. Gastroenterol. Hepatol. 4, 27 (2019).
Strobel, O., Neoptolemos, J., Jager, D. & Buchler, M. W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 16, 11–26 (2019).
Neoptolemos, J. P. et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 350, 1200–1210 (2004).
Oettle, H. et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310, 1473–1481 (2013).
Neoptolemos, J. P. et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304, 1073–1081 (2010).
Neoptolemos, J. P. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024 (2017).
Conroy, T. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379, 2395–2406 (2018).
Frei, E. III Clinical cancer research: an embattled species. Cancer 50, 1979–1992 (1982).
Quezada-Diaz, F. F. & Smith, J. J. Neoadjuvant therapy for rectal cancer. Surg. Oncol. Clin. N. Am. 31, 279–291 (2022).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355, 11–20 (2006).
Al-Batran, S. E. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393, 1948–1957 (2019).
Seufferlein, T. et al. Perioperative or only adjuvant gemcitabine plus nab-paclitaxel for resectable pancreatic cancer (NEONAX) – a randomized phase II trial of the AIO pancreatic cancer group. Ann. Oncol. https://doi.org/10.1016/j.annonc.2022.09.161 (2022).
Asbun, H. J. et al. When to perform a pancreatoduodenectomy in the absence of positive histology? A consensus statement by the International Study Group of Pancreatic Surgery. Surgery 155, 887–892 (2014).
National Comprehensive Cancer Network. NCCN guidelines: V1.2022, Pancreatic adenocarcinoma. NCCN https://www.nccn.org/guidelines/category_1 (2022).
Ghaneh, P. et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy, in patients with borderline resectable pancreatic cancer (ESPAC5): a four arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 8, 157–168 (2023).
Fietkau, R. et al. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresectable locally advanced pancreatic cancer: first results of the CONKO-007 trial [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 4008 (2022).
Amin, M. B. et al. (eds) AJCC Cancer Staging Manual 8th edn (Springer, 2017).
Callery, M. P. et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann. Surg. Oncol. 16, 1727–1733 (2009).
Varadhachary, G. R. et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann. Surg. Oncol. 13, 1035–1046 (2006).
Katz, M. H. et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 151, e161137 (2016).
Ishikawa, O. et al. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann. Surg. 215, 231–236 (1992).
Nakao, A. et al. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann. Surg. 255, 103–108 (2012).
Katz, M. H. et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann. Surg. Oncol. 20, 2787–2795 (2013).
Kirkegard, J. et al. Intra-observer agreements in multidisciplinary team assessments of pancreatic cancer patients. J. Surg. Oncol. 124, 1402–1408 (2021).
Ahmad, S. A. et al. Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX versus gemcitabine/Nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann. Surg. 272, 481–486 (2020).
Sohal, D. P. S. et al. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 7, 421–427 (2021).
Katz, M. H. G. et al. Efficacy of preoperative mFOLFIRINOX vs mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: the A021501 phase 2 randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2022.2319 (2022).
Douglas, J. E. et al. PIONEER-Panc: a platform trial for phase II randomized investigations of new and emerging therapies for localized pancreatic cancer. BMC Cancer 22, 14 (2022).
Katz, M. H. et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J. Am. Coll. Surg. 206, 833–846 (2008).
Isaji, S. et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 18, 2–11 (2018).
Hayasaki, A. et al. Survival analysis in patients with pancreatic ductal adenocarcinoma undergoing chemoradiotherapy followed by surgery according to the international consensus on the 2017 definition of borderline resectable cancer. Cancers 10, 65 (2018).
Medrano, J. et al. Patient outcome according to the 2017 international consensus on the definition of borderline resectable pancreatic ductal adenocarcinoma. Pancreatology 20, 223–228 (2020).
Springfeld, C. et al. Chemotherapy for pancreatic cancer. Presse Med. 48, e159–e174 (2019).
Burris, H. A. III et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413 (1997).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Cunningham, D. et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 27, 5513–5518 (2009).
Tempero, M. A. et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 4000 (2019).
Reni, M. et al. A randomized phase II trial of two different 4-drug combinations in advanced pancreatic adenocarcinoma: cisplatin, capecitabine, gemcitabine plus either epirubicin or docetaxel (PEXG or PDXG regimen). Cancer Chemother. Pharmacol. 69, 115–123 (2012).
Breakstone, R. et al. The Brown University Oncology Group experience with FOLFOX + nab-paclitaxel [FOLFOX-A] for metastatic and locally advanced pancreatic, BrUOG-292 and BrUOG-318. Am. J. Clin. Onc. 45, 327–332 (2022).
Jameson, G. S. et al. Response rate following albumin-bound paclitaxel plus gemcitabine plus cisplatin treatment among patients with advanced pancreatic cancer: a phase 1b/2 pilot clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.3394 (2019).
Carrato, A. et al. Sequential nab-paclitaxel/gemcitabine followed by modified FOLFOX for first-line metastatic pancreatic cancer: the SEQUENCE trial [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 4022 (2022).
Wainberg, Z. A. et al. NAPOLI-3: a randomized, open-label phase 3 study of liposomal irinotecan + 5-fluorouracil/leucovorin + oxaliplatin (NALIRIFOX) versus nab-paclitaxel + gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) [abstract]. J. Clin. Oncol. 41 (Suppl. 4), LBA661 (2023).
Seifert, L. et al. Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology 150, 1659–1672.e5 (2016).
Hammel, P. et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 315, 1844–1853 (2016).
Nolan, E. et al. Radiation exposure elicits a neutrophil-driven response in healthy lung tissue that enhances metastatic colonization. Nat. Cancer 3, 173–187 (2022).
Versteijne, E. et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 38, 1763–1773 (2020).
Versteijne, E. et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J. Clin. Oncol. 40, 1220–1230 (2022).
Springfeld, C. & Neoptolemos, J. P. The role of neoadjuvant therapy for resectable pancreatic cancer remains uncertain. Nat. Rev. Clin. Oncol. 19, 285–286 (2022).
Jang, J. Y. et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann. Surg. 268, 215–222 (2018).
Reyngold, M. et al. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 7, 735–738 (2021).
Valle, J. W. et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J. Clin. Oncol. 32, 504–512 (2014).
Schwarz, L. et al. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy: a multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study) [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 4134 (2022).
Schwarz, L. et al. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy - a multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study. BMC Cancer 18, 762 (2018).
Halloran, C. M. et al. A multicenter, randomized, double-blinded, clinical trial comparing Cattell–Warren and Blumgart anastomoses following partial pancreatoduodenectomy: PANasta trial. Ann. Surg. Open 3, e198 (2022).
Xu, Z. et al. Clinical impact of molecular subtyping of pancreatic cancer. Front. Cell Dev. Biol. 9, 743908 (2021).
O’Reilly, E. M. et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J. Clin. Oncol. 38, 1378–1388 (2020).
Hofmann, M. H., Gerlach, D., Misale, S., Petronczki, M. & Kraut, N. Expanding the reach of precision oncology by drugging all KRAS mutants. Cancer Discov. 12, 924–937 (2022).
Bekaii-Saab, T. S. et al. KRYSTAL-1: updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation [abstract]. J. Clin. Oncol. 40 (Suppl. 4), 519 (2022).
Philip, P. A. et al. Molecular characterization of KRAS wild-type tumors in patients with pancreatic adenocarcinoma. Clin. Cancer Res. 28, 2704–2714 (2022).
Allen, M. J. et al. Molecular characterisation of pancreatic ductal adenocarcinoma with NTRK fusions and review of the literature. J. Clin. Pathol. https://doi.org/10.1136/jclinpath-2021-207781 (2021).
Heining, C. et al. NRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov. 8, 1087–1095 (2018).
Thein, K. Z. et al. Identification of KRAS(G12C) mutations in circulating tumor DNA in patients with cancer. JCO Precis. Oncol. 6, e2100547 (2022).
Cercek, A. et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 386, 2363–2376 (2022).
Marabelle, A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10 (2020).
Bockorny, B., Grossman, J. E. & Hidalgo, M. Facts and hopes in immunotherapy of pancreatic cancer. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-21-3452 (2022).
Perri, G. et al. Response and survival associated with first-line FOLFIRINOX vs gemcitabine and nab-paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. JAMA Surg. 155, 832–839 (2020).
Heger, U. et al. Induction chemotherapy in pancreatic cancer: CA 19-9 may predict resectability and survival. HPB 22, 224–232 (2020).
Allenson, K. et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 28, 741–747 (2017).
Bernard, V. et al. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology 156, 108–118.e4 (2019).
Yin, L. et al. Improved assessment of response status in patients with pancreatic cancer treated with neoadjuvant therapy using somatic mutations and liquid biopsy analysis. Clin. Cancer Res. 27, 740–748 (2021).
Botta, G. P. et al. Association of personalized and tumor-informed ctDNA with patient survival outcomes in pancreatic adenocarcinoma [abstract]. J. Clin. Oncol. 40 (Suppl. 4), 517 (2022).
Campbell, F., Cairns, A., Duthie, F. & Feakins, R. Dataset for Histopathological Reporting of Carcinomas of the Pancreas, Ampulla of Vater and Common Bile Duct (The Royal College of Pathologists, 2019).
Katz, M. H. et al. Standardization of surgical and pathologic variables is needed in multicenter trials of adjuvant therapy for pancreatic cancer: results from the ACOSOG Z5031 trial. Ann. Surg. Oncol. 18, 337–344 (2011).
Verbeke, C., Haberle, L., Lenggenhager, D. & Esposito, I. Pathology assessment of pancreatic cancer following neoadjuvant treatment: time to move on. Pancreatology 18, 467–476 (2018).
Washington, K. et al. Protocol for the Examination of Specimens from Patients with Carcinoma of the Pancreas (College of American Pathologists, 2016).
Groot, V. P. et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann. Surg. 267, 936–945 (2018).
Motoi, F. et al. A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J. Gastroenterol. 54, 194–203 (2019).
Janssen, Q. P. et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer 21, 300 (2021).
Labori, K. J. et al. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial - 1 (NorPACT-1)) – study protocol for a national multicentre randomized controlled trial. BMC Surg. 17, 94 (2017).
Heinrich, S. et al. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer 11, 346 (2011).
Al-Batran, S.-E. et al. Randomized multicenter phase II/III study with adjuvant gemcitabine versus neoadjuvant/adjuvant FOLFIRINOX in resectable pancreatic cancer: the NEPAFOX trial [abstract]. J. Clin. Oncol. 39 (Suppl. 3), 406 (2021).
Golcher, H. et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther. Onkol. 191, 7–16 (2015).
Casadei, R. et al. Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: a single-center prospective, randomized, controlled trial which failed to achieve accrual targets. J. Gastrointest. Surg. 19, 1802–1812 (2015).
Motoi, F. et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 49, 190–194 (2019).
Uesaka, K. et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388, 248–257 (2016).
Okusaka, T. et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a synopsis. Pancreas 49, 326–335 (2020).
Hall, W. A. et al. Value of neoadjuvant radiation therapy in the management of pancreatic adenocarcinoma. J. Clin. Oncol. 39, 3773–3777 (2021).
Bahadoer, R. R. et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 22, 29–42 (2021).
Barrord, M. et al. Patterns of failure after neoadjuvant stereotactic body radiation therapy or fractionated chemoradiation in resectable and borderline resectable pancreatic cancer. Pancreas 49, 941–946 (2020).
Murphy, J. E. et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 4, 963–969 (2018).
Sanford, N. N., Yeap, B. Y. & Hong, T. S. Measuring the effect of local control from radiotherapy in patients with pancreatic adenocarcinoma. JAMA Oncol. 8, 337–338 (2022).
Kunzmann, V. et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 6, 128–138 (2021).
Hackert, T. et al. Locally advanced pancreatic cancer: neoadjuvant therapy with Folfirinox results in resectability in 60% of the patients. Ann. Surg. 264, 457–463 (2016).
Suker, M. et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 17, 801–810 (2016).
Philip, P. A. et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol. Hepatol. 5, 285–294 (2020).
Ferrone, C. R. et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 261, 12–17 (2015).
Hartwig, W. et al. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann. Surg. Oncol. 20, 2188–2196 (2013).
Cai, B. et al. Sub-adventitial divestment technique for resecting artery-involved pancreatic cancer: a retrospective cohort study. Langenbecks Arch. Surg. 406, 691–701 (2021).
Diener, M. K. et al. Periarterial divestment in pancreatic cancer surgery. Surgery 169, 1019–1025 (2021).
Klompmaker, S. et al. Distal pancreatectomy with celiac axis resection (DP-CAR) for pancreatic cancer. How I do it. J. Gastrointest. Surg. 22, 1804–1810 (2018).
Klotz, R. et al. The TRIANGLE operation for pancreatic head and body cancers: early postoperative outcomes. HPB 24, 332–341 (2022).
Schneider, M., Hackert, T., Strobel, O. & Buchler, M. W. Technical advances in surgery for pancreatic cancer. Br. J. Surg. 108, 777–785 (2021).
Habib, J. R. et al. Periadventitial dissection of the superior mesenteric artery for locally advanced pancreatic cancer: surgical planning with the “halo sign” and “string sign”. Surgery 169, 1026–1031 (2021).
Hackert, T. et al. Portal vein resection in pancreatic cancer surgery: risk of thrombosis and radicality determine survival. Ann. Surg. https://doi.org/10.1097/SLA.0000000000005444 (2022).
Loos, M. et al. Arterial resection in pancreatic cancer surgery: effective after a learning curve. Ann. Surg. 275, 759–768 (2022).
Bachellier, P., Addeo, P., Faitot, F., Nappo, G. & Dufour, P. Pancreatectomy with arterial resection for pancreatic adenocarcinoma: how can it be done safely and with which outcomes?: a single institution’s experience with 118 patients. Ann. Surg. 271, 932–940 (2020).
Hackert, T. et al. Radical surgery of oligometastatic pancreatic cancer. Eur. J. Surg. Oncol. 43, 358–363 (2017).
Tachezy, M. et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 160, 136–144 (2016).
Hank, T. et al. Oncological outcome of conversion surgery after preoperative chemotherapy for metastatic pancreatic cancer. Ann. Surg. https://doi.org/10.1097/SLA.0000000000005481 (2022).
Jones, R. P. et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surg. 154, 1038–1048 (2019).
Homma, Y. et al. Outcomes of lung metastasis from pancreatic cancer: a nationwide multicenter analysis. J. Hepatobiliary Pancreat. Sci. 29, 552–561 (2022).
Gebauer, F. et al. Study protocol of an open-label, single arm phase II trial investigating the efficacy, safety and quality of life of neoadjuvant chemotherapy with liposomal irinotecan combined with oxaliplatin and 5-fluorouracil/folinic acid followed by curative surgical resection in patients with hepatic oligometastatic adenocarcinoma of the pancreas (HOLIPANC). BMC Cancer 21, 1239 (2021).
AIO-Studien. AIO-PAK-0219xx: Intensified treatment in patients with local operable but oligometastatic pancreatic cancer–multimodal surgical treatment versus systemic chemotherapy alone: a randomized controlled phase 3 trial (METAPANC). AIO-Studien www.aio-portal.de/files/content/studien/studiendatenbank/AIO-PAK-0219_s.pdf (2021).
Klaiber, U. et al. Prognostic factors of survival after neoadjuvant treatment and resection for initially unresectable pancreatic cancer. Ann. Surg. 273, 154–162 (2021).
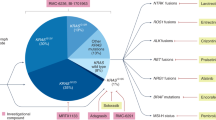
Connor, A. A. & Gallinger, S. Pancreatic cancer evolution and heterogeneity: integrating omics and clinical data. Nat. Rev. Cancer 22, 131–142 (2022).
Strobel, O. et al. Actual five-year survival after upfront resection for pancreatic ductal adenocarcinoma: who beats the odds? Ann. Surg. 275, 962–971 (2022).
Collisson, E. A., Bailey, P., Chang, D. K. & Biankin, A. V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 16, 207–220 (2019).
Moffitt, R. A. et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 47, 1168–1178 (2015).
Bailey, P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016).
Beatty, G. L., Werba, G., Lyssiotis, C. A. & Simeone, D. M. The biological underpinnings of therapeutic resistance in pancreatic cancer. Genes. Dev. 35, 940–962 (2021).
Chan-Seng-Yue, M. et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 52, 231–240 (2020).
Shen, S., Vagner, S. & Robert, C. Persistent cancer cells: the deadly survivors. Cell 183, 860–874 (2020).
Grunwald, B. T. et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 184, 5577–5592.e18 (2021).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
Zhou, X. et al. Persister cell phenotypes contribute to poor patient outcomes after neoadjuvant chemotherapy in PDAC [abstract]. Cancer Res. 82 (Suppl. 22), A010 (2022).
Aung, K. L. et al. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin. Cancer Res. 24, 1344–1354 (2018).
Porter, R. L. et al. Epithelial to mesenchymal plasticity and differential response to therapies in pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1914915116 (2019).
Hwang, W. L. et al. Single-nucleus and spatial transcriptome profiling of pancreatic cancer identifies multicellular dynamics associated with neoadjuvant treatment. Nat. Genet. 54, 1178–1191 (2022).
Maeda, S. et al. Pathological treatment response has different prognostic implications for pancreatic cancer patients treated with neoadjuvant chemotherapy or chemoradiotherapy. Surgery 171, 1379–1387 (2022).
Schreyer, D., Neoptolemos, J. P., Barry, S. T. & Bailey, P. Deconstructing pancreatic cancer using next generation-omic technologies-from discovery to knowledge-guided platforms for better patient management. Front. Cell Dev. Biol. 9, 795735 (2021).
Nicolle, R. et al. A transcriptomic signature to predict adjuvant gemcitabine sensitivity in pancreatic adenocarcinoma. Ann. Oncol. 32, 250–260 (2021).
Greenhalf, W. et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J. Natl Cancer Inst. 106, djt347 (2014).
Golan, T. et al. Genomic features and classification of homologous recombination deficient pancreatic ductal adenocarcinoma. Gastroenterology 160, 2119–2132.e9 (2021).
Kaissis, G. et al. A machine learning algorithm predicts molecular subtypes in pancreatic ductal adenocarcinoma with differential response to gemcitabine-based versus FOLFIRINOX chemotherapy. PLoS ONE 14, e0218642 (2019).
Dean, A. et al. Dual αV-integrin and neuropilin-1 targeting peptide CEND-1 plus nab-paclitaxel and gemcitabine for the treatment of metastatic pancreatic ductal adenocarcinoma: a first-in-human, open-label, multicentre, phase 1 study. Lancet Gastroenterol. Hepatol. 7, 943–951 (2022).
Ullman, N. A., Burchard, P. R., Dunne, R. F. & Linehan, D. C. Immunologic strategies in pancreatic cancer: making cold tumors hot. J. Clin. Oncol. 40, 2789–2805 (2022).
Balachandran, V. P. et al. Phase I trial of adjuvant autogene cevumeran, an individualized mRNA neoantigen vaccine, for pancreatic ductal adenocarcinoma [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 2516 (2022).
Fietkau, R. et al. R0 resection following chemo (radio)therapy improves survival of primary inoperable pancreatic cancer patients. Interim results of the German randomized CONKO-007+/− trial. Strahlenther. Onkol. 197, 8–18 (2021).
Reni, M. et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol. Hepatol. 3, 413–423 (2018).
Mukherjee, S. et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 14, 317–326 (2013).
Strickler, J. H. et al. Sotorasib in KRAS p.G12C-mutated advanced pancreatic cancer. N. Engl. J. Med. 88, 33–43 (2023).
Kalser, M. H. & Ellenberg, S. S. Pancreatic cancer. adjuvant combined radiation and chemotherapy following curative resection. Arch. Surg. 120, 899–903 (1985).
Bakkevold, K. E., Arnesjo, B., Dahl, O. & Kambestad, B. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater – results of a controlled, prospective, randomised multicentre study. Eur. J. Cancer 29A, 698–703 (1993).
Takada, T. et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 95, 1685–1695 (2002).
Klinkenbijl, J. H. et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann. Surg. 230, 776–782 (1999).
Smeenk, H. G. et al. Adjuvant 5-FU-based chemoradiotherapy for patients undergoing R-1/R-2 resections for pancreatic cancer. Dig. Surg. 22, 321–328 (2005).
Neoptolemos, J. P. et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 358, 1576–1585 (2001).
Oettle, H. et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297, 267–277 (2007).
Regine, W. F. et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 299, 1019–1026 (2008).
Regine, W. F. et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann. Surg. Oncol. 18, 1319–1326 (2011).
Ueno, H. et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br. J. Cancer 101, 908–915 (2009).
Schmidt, J. et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J. Clin. Oncol. 30, 4077–4083 (2012).
Sinn, M. et al. CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after r0 resection of pancreatic cancer: a multicenter randomized phase III trial. J. Clin. Oncol. 35, 3330–3337 (2017).
Sinn, M. et al. CONKO-006: a randomised double-blinded phase IIb-study of additive therapy with gemcitabine + sorafenib/placebo in patients with R1 resection of pancreatic cancer–final results. Eur. J. Cancer 138, 172–181 (2020).
Abrams, R. A. et al. Results of the NRG Oncology/RTOG 0848 adjuvant chemotherapy question-erlotinib+gemcitabine for resected cancer of the pancreatic head: a phase II randomized clinical trial. Am. J. Clin. Oncol. 43, 173–179 (2020).
Tempero, M. et al. Phase 3 APACT trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P + Gem) vs gemcitabine (Gem) alone in patients with resected pancreatic cancer (PC): updated 5-year overall survival [abstract LBA-1]. Ann. Oncol. 32 (Suppl. 3), S226 (2021).
Unno, M. et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05) [abstract]. J. Clin. Oncol. 37 (Suppl. 4), 189 (2019).
Yamaguchi, J. et al. Results of a phase II study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab-PTX) for borderline-resectable pancreatic cancer (NUPAT-01). Ann. Surg. 275, 1043–1049 (2022).
Ducreux, M. P. et al. PRODIGE 29-UCGI 26(NEOPAN): a phase III randomised trial comparing chemotherapy with folfirinox or gemcitabine in locally advanced pancreatic carcinoma (LAPC) [abstract 1296MO]. Ann. Oncol. 33 (Suppl. 7), S1136 (2022).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
C.S. has acted as an adviser to AstraZeneca, Bayer, Eisai, lncyte, MSD, Roche and Servier. P.A.P. has received honoraria and/or acted as a consultant to AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi, Guardant, Incyte, Ipsem, Merck, Servier, Tempus and Trisalus, has received research support from Caris, Karyopharm, Novocure and Rafael, and is a member of data safety monitoring boards for Cyclacel, Erytech and Oncolytics. T.H. has acted as an adviser and/or consultant to Boston Scientific, GSK, lnviata, the Lustgarten Foundation, Merck, Novocure and Pan Ther Therapeutics, and has received research funding from AstraZeneca, BMS, GSK, lntraOp, lpsen and Taiho. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks S Isaji, J. Windsor and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Springfeld, C., Ferrone, C.R., Katz, M.H.G. et al. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol 20, 318–337 (2023). https://doi.org/10.1038/s41571-023-00746-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00746-1
This article is cited by
-
Therapeutic developments in pancreatic cancer
Nature Reviews Gastroenterology & Hepatology (2024)
-
ALYREF-JunD-SLC7A5 axis promotes pancreatic ductal adenocarcinoma progression through epitranscriptome-metabolism reprogramming and immune evasion
Cell Death Discovery (2024)
-
Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma
Nature Reviews Clinical Oncology (2024)
-
Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer
Nature Reviews Gastroenterology & Hepatology (2024)
-
DDX3X interacts with SIRT7 to promote PD-L1 expression to facilitate PDAC progression
Oncogenesis (2024)