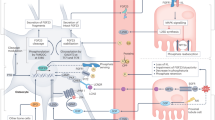
Abstract
Phosphate is critical for the maintenance of skeletal integrity, is a necessary component of important biomolecules, and is central to signal transduction and cell metabolism. It is becoming clear that endocrine communication between the skeleton, kidney, and the intestine is involved in maintaining appropriate serum phosphate concentrations, and that the kidney is the primary site for minute-to-minute regulation of phosphate levels. The identification of genetic alterations in Mendelian disorders of hypophosphatemia and hyperphosphatemia has led to the isolation of novel genes and the identification of new roles for existing proteins—such as fibroblast growth factor 23 and its processing systems, the co-receptor α-klotho, and phosphate transporters—in the control of renal phosphate handling. Recent findings also indicate that fibroblast growth factor 23 has feedback mechanisms involving parathyroid hormone and vitamin D that control phosphate homeostasis. This Review will highlight genetic, in vitro and in vivo findings, and will discuss how these clinical and experimental discoveries have uncovered novel aspects of renal phosphate handling and opened new research and therapeutic avenues.
Key Points
-
Fibroblast growth factor 23 (FGF23) is a phosphaturic hormone produced in bone
-
FGF23 and parathyroid hormone (PTH) decrease the expression of the sodium–phosphate co-transporters Npt2a and Npt2c
-
FGF23 and PTH have opposing effects on 1,25-dihydroxyvitamin D, with FGF23 suppressing its production, and PTH increasing its production
-
The actions of FGF23 in the kidney are dependent on the expression of the co-receptor klotho, but the mechanisms guiding FGF23-dependent signaling may be complex
-
Human disorders associated with increased circulating concentrations of FGF23 are characterized by hypophosphatemia, and disorders associated with reduced FGF23 bioactivity are characterized by hyperphosphatemia
-
FGF23 and its receptor systems are promising targets for targeted therapeutics in the treatment of both heritable and acquired disorders of phosphate handling
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Takeda, E., Taketani, Y., Sawada, N., Sato, T. & Yamamoto, H. The regulation and function of phosphate in the human body. Biofactors 21, 345–355 (2004).
Tenenhouse, H. S. Regulation of phosphorus homeostasis by the type iia Na/phosphate cotransporter. Annu. Rev. Nutr. 25, 197–214 (2005).
Greenberg, B. G., Winters, R. W. & Graham, J. B. The normal range of serum inorganic phosphorus and its utility as a discriminant in the diagnosis of congenital hypophosphatemia. J. Clin. Endocrinol. Metab. 20, 364–379 (1960).
Burritt, M. F. et al. Pediatric reference intervals for 19 biologic variables in healthy children. Mayo Clin. Proc. 65, 329–336 (1990).
Walton, J. & Gray, T. K. Absorption of inorganic phosphate in the human small intestine. Clin. Sci. (Lond.) 56, 407–412 (1979).
Corut, A. et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am. J. Hum. Genet. 79, 650–656 (2006).
Sabbagh, Y. et al. Intestinal Npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 20, 2348–2358 (2009).
Berndt, T. et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc. Natl Acad. Sci. USA 104, 11085–11090 (2007).
Silve, C. & Friedlander, G. in The Kidney: Physiology & Pathophysiology (eds Seldin, D. W. & Giebisch, G.) 1885–1904 (Lippincott Williams & Wilkins, Philadelphia, 2000).
Baron, R. (ed.) Anatomy and Biology of Bone Matrix and Cellular Elements (American Society for Bone and Mineral Research, Washington, DC, 2003).
Beck, L. et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl Acad. Sci. USA 95, 5372–5377 (1998).
Ohkido, I., Segawa, H., Yanagida, R., Nakamura, M. & Miyamoto, K. Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflugers Arch. 446, 106–115 (2003).
Lorenz-Depiereux, B. et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am. J. Hum. Genet. 78, 193–201 (2006).
Bergwitz, C. et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 78, 179–192 (2006).
Omdahl, J. L., Gray, R. W., Boyle, I. T., Knutson, J. & DeLuca, H. F. Regulation of metabolism of 25-hydroxycholecalciferol by kidney tissue in vitro by dietary calcium. Nat. New Biol. 237, 63–64 (1972).
Parfitt, A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part IV of IV parts: the state of the bones in uremic hyperaparathyroidism—the mechanisms of skeletal resistance to PTH in renal failure and pseudohypoparathyroidism and the role of PTH in osteoporosis, osteopetrosis, and osteofluorosis. Metabolism 25, 1157–1188 (1976).
Bacic, D. et al. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 69, 495–503 (2006).
Pfister, M. F. et al. Parathyroid hormone-dependent degradation of type II Na+/Pi cotransporters. J. Biol. Chem. 272, 20125–20130 (1997).
Lotscher, M. et al. New aspects of adaptation of rat renal Na-Pi cotransporter to alterations in dietary phosphate. Kidney Int. 49, 1012–1018 (1996).
Takahashi, F. et al. Effects of dietary Pi on the renal Na+-dependent Pi transporter NaPi-2 in thyroparathyroidectomized rats. Biochem. J. 333, 175–181 (1998).
Traebert, M., Volkl, H., Biber, J., Murer, H. & Kaissling, B. Luminal and contraluminal action of 1–34 and 3–34 PTH peptides on renal type IIa Na-P(i) cotransporter. Am. J. Physiol. Renal Physiol. 278, F792–F798 (2000).
Bacic, D. et al. Involvement of the MAPK-kinase pathway in the PTH-mediated regulation of the proximal tubule type IIa Na+/Pi cotransporter in mouse kidney. Pflugers Arch. 446, 52–60 (2003).
Quarles, L. D. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 118, 3820–3828 (2008).
ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 (2000).
White, K. E., Larsson, T. E. & Econs, M. J. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr. Rev. 27, 221–241 (2006).
Riminucci, M. et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Invest. 112, 683–692 (2003).
Shimada, T. et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143, 3179–3182 (2002).
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Goetz, R. et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc. Natl Acad. Sci. USA (2009).
Perwad, F. et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1, 25-dihydroxyvitamin D metabolism in mice. Endocrinology 146, 5358–5364 (2005).
Antoniucci, D. M., Yamashita, T. & Portale, A. A. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J. Clin. Endocrinol. Metab. 91, 3144–3149 (2006).
Nishida, Y. et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 70, 2141–2147 (2006).
Ito, N. et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J. Bone Miner. Metab. 25, 419–422 (2007).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci USA 98, 6500–6505 (2001).
Larsson, T. et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145, 3087–3094 (2004).
Shimada, T. et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem. Biophys. Res. Commun. 314, 409–414 (2004).
Kolek, O. I. et al. 1α, 25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G1036–G1042 (2005).
Liu, S. et al. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol. Endocrinol. 23, 1505–1518 (2009).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004).
Shimada, T. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004).
Sitara, D. et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23, 421–432 (2004).
Hesse, M., Frohlich, L. F., Zeitz, U., Lanske, B. & Erben, R. G. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 26, 75–84 (2007).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Ichikawa, S. et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 117, 2684–2691 (2007).
Benet-Pages, A., Orlik, P., Strom, T. M. & Lorenz-Depiereux, B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 14, 385–390 (2005).
Topaz, O. et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 36, 579–581 (2004).
Ichikawa, S. et al. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology 150, 2543–2550 (2009).
Garringer, H. J. et al. The role of mutant UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J. Clin. Endocrinol. Metab. 91, 4037–4042 (2006).
Ohnishi, M., Nakatani, T., Lanske, B. & Razzaque, M. S. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 75, 1166–1172 (2009).
Schouten, B. J., Hunt, P. J., Livesey, J. H., Frampton, C. M. & Soule, S. G. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J. Clin. Endocrinol. Metab. 94, 2332–2337 (2009).
Sato, K. et al. Saccharated ferric oxide (SFO)-induced osteomalacia: in vitro inhibition by SFO of bone formation and 1, 25-dihydroxy-vitamin D production in renal tubules. Bone 21, 57–64 (1997).
Sato, K. & Shiraki, M. Saccharated ferric oxide-induced osteomalacia in Japan: iron-induced osteopathy due to nephropathy. Endocr. J. 45, 431–439 (1998).
Schouten, B. J., Doogue, M. P., Soule, S. G. & Hunt, P. J. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann. Clin. Biochem. 46, 167–169 (2009).
Shimizu, Y. et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone 45, 814–816 (2009).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006).
Aono, Y. et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J. Bone Miner. Res. 24, 1879–1888 (2009).
Segawa, H. et al. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am. J. Physiol. Renal Physiol. 292, F769–F779 (2007).
Brownstein, C. A. et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc. Natl Acad. Sci. USA 105, 3455–3460 (2008).
Liu, S., Vierthaler, L., Tang, W., Zhou, J. & Quarles, L. D. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J. Am. Soc. Nephrol. 19, 2342–2350 (2008).
Matsumura, Y. et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 242, 626–630 (1998).
Imura, A. et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 565, 143–147 (2004).
Farrow, E. G., Davis, S. I., Summers, L. J. & White, K. E. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J. Am. Soc. Nephrol. 20, 955–960 (2009).
Kurosu, H. & Kuro, O. M. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol. Cell. Endocrinol. 299, 72–78 (2009).
Krajisnik, T. et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 195, 125–131 (2007).
Ben-Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Brown, W. W. et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen's metaphyseal chondrodysplasia. J. Clin. Endocrinol. Metab. 94, 17–20 (2009).
Rhee, Y. et al. FGF23 gene expression is upregulated by PTH receptor activation in osteocytes in vitro and in vivo: a parathyroid-bone link influencing the endocrine function of osteocytes [abstract]. J. Bone Miner. Res. 24, (2009).
O'Brien, C. A. et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One 3, e2942 (2008).
Kobayashi, K. et al. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur. J. Endocrinol. 154, 93–99 (2006).
Econs, M. J. & McEnery, P. T. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J. Clin. Endocrinol. Metab. 82, 674–681 (1997).
Imel, E. A., Hui, S. L. & Econs, M. J. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J. Bone Miner. Res. 22, 520–526 (2007).
Sabbagh, Y., Tenenhouse, H. S. & Econs, M. J. Mendelian hypophosphatemias. In The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R. et al.) Ch. 197 (McGraw Hill, New York, 2008).
Tenenhouse, H. S., Roy, S., Martel, J. & Gauthier, C. Differential expression, abundance, and regulation of Na+-phosphate cotransporter genes in murine kidney. Am. J. Physiol. 275, F527–F534 (1998).
[No authors listed] A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat. Genet. 11, 130–136 (1995).
Ichikawa, S. et al. Mutational survey of the PHEX gene in patients with X-linked hypophosphatemic rickets. Bone 43, 663–666 (2008).
Beck, L. et al. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J. Clin. Invest. 99, 1200–1209 (1997).
Jonsson, K. B. et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 348, 1656–1663 (2003).
Weber, T. J., Liu, S., Indridason, O. S. & Quarles, L. D. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J. Bone Miner. Res. 18, 1227–1234 (2003).
Yamazaki, Y. et al. Elevated circulatory and expression level of fibroblast growth factor (FGF)-23 in hypophosphatemic mice. Bone 32, S88 (2003).
Aono, Y. et al. The neutralization of FGF-23 ameliorates hypophosphatemia and rickets in Hyp mice. J. Bone Miner. Metab. 23, 1509–1518 (2003).
Liu, S. et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 278, 37419–37426 (2003).
Tenenhouse, H. S. & Beck, L. Renal Na+-phosphate cotransporter gene expression in X-linked Hyp and Gy mice. Kidney Int. 49, 1027–1032 (1996).
Feng, J. Q. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 (2006).
Lorenz-Depiereux, B. et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 38, 1248–1250 (2006).
Fisher, L. W. & Fedarko, N. S. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect. Tissue Res. 44 (Suppl. 1), 33–40 (2003).
Feng, J. Q. et al. The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J. Dent. Res. 82, 776–780 (2003).
Ling, Y. et al. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J. Bone Miner. Res. 20, 2169–2177 (2005).
Yuan, B. et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J. Clin. Invest. 118, 722–734 (2008).
Folpe, A. L. et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am. J. Surg. Pathol. 28, 1–30 (2004).
Ryan, E. A. & Reiss, E. Oncogenous osteomalacia: review of the world literature of 42 cases and report of two new cases. Am. J. Med. 77, 501–512 (1984).
Econs, M. J. & Drezner, M. K. Tumor-induced osteomalacia—unveiling a new hormone. N. Engl. J. Med. 330, 1679–1681 (1994).
White, K. E. et al. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J. Clin. Endocrinol. Metab. 86, 497–500 (2001).
Jan De Beur, S. M. et al. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J. Bone Miner. Res. 17, 1102–1110 (2002).
Yamazaki, Y. et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 87, 4957–4960 (2002).
Chalew, S. A., Lovchik, J. C., Brown, C. M. & Sun, C. C. Hypophosphatemia induced in mice by transplantation of a tumor-derived cell line from a patient with oncogenic rickets. J. Pediatr. Endocrinol. Metab. 9, 593–597 (1996).
Tieder, M. et al. Hereditary hypophosphatemic rickets with hypercalciuria. N. Engl. J. Med. 312, 611–617 (1985).
Jaureguiberry, G., Carpenter, T. O., Forman, S., Juppner, H. & Bergwitz, C. A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am. J. Physiol. Renal Physiol. 295, F371–F379 (2008).
Levi, M. Novel NaPi-2c mutations that cause mistargeting of NaPi-2c protein and uncoupling of Na-Pi cotransport cause HHRH. Am. J. Physiol. Renal Physiol. 295, F369–F370 (2008).
Segawa, H. et al. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J. Am. Soc. Nephrol. 20, 104–113 (2008).
Prie, D. et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N. Engl. J. Med. 347, 983–991 (2002).
Khundmiri, S. J. et al. Novel regulatory function for NHERF-1 in Npt2a transcription. Am. J. Physiol. Renal Physiol. 294, F840–F849 (2008).
Inclan, A., Leon, P. & Camejo, M. G. Tumoral calcinosis. JAMA 121, 490–495 (1943).
Kato, K. et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 281, 18370–18377 (2006).
Benet-Pages, A. et al. FGF23 is processed by proprotein convertases but not by PHEX. Bone 35, 455–462 (2004).
Garringer, H. J. et al. Two novel GALNT3 mutations in familial tumoral calcinosis. Am. J. Med. Genet. A 143, 2390–2396 (2007).
Larsson, T. et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J. Clin. Endocrinol. Metab. 90, 2424–2427 (2005).
Bergwitz, C. et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J. Clin. Endocrinol. Metab. 94, 4267–4274 (2009).
Frishberg, Y. et al. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J. Mol. Med. 83, 33–38 (2005).
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047 (2007).
Silver, J., Kilav, R., Sela-Brown, A. & Naveh-Many, T. Molecular mechanisms of secondary hyperparathyroidism. Pediatr. Nephrol. 14, 626–628 (2000).
Larsson, T., Nisbeth, U., Ljunggren, O., Juppner, H. & Jonsson, K. B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 64, 2272–2279 (2003).
Gutierrez, O. M. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 359, 584–592 (2008).
The Indiana Clinical and Translational Trials Unit. A phase I, double-blind, randomized, placebo-controlled, single-dose, dose-escalation study of KRN23 in X-linked hypophosphatemia patients [online], (2009).
Acknowledgements
The authors would like to acknowledge support from NIH grant DK063934 (K. E. White), the Showalter Foundation, Genzyme Corporation, and the Indiana Genomics Initiative (INGEN) of Indiana University, supported in part by the Lilly Endowment. The authors would also like to acknowledge the editorial and scientific contributions of Ms L. J. Summers, Indiana University School of Medicine, Indianapolis, IN, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
K. E. White declares associations with the following companies: Genzyme (grant/research support), Kyowa Hakko Kirin (royalties for licensing the FGF23 gene). E. G. Farrow declares no competing interests.
Rights and permissions
About this article
Cite this article
Farrow, E., White, K. Recent advances in renal phosphate handling. Nat Rev Nephrol 6, 207–217 (2010). https://doi.org/10.1038/nrneph.2010.17
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.17
This article is cited by
-
The human pathogenic 91del7 mutation in SLC34A1 has no effect in mineral homeostasis in mice
Scientific Reports (2022)
-
Regulation of FGF23: Beyond Bone
Current Osteoporosis Reports (2021)
-
Phosphate as a Signaling Molecule
Calcified Tissue International (2021)
-
Interaction between serum FGF-23 and PTH in renal phosphate excretion, a case-control study in hypoparathyroid patients
BMC Nephrology (2020)
-
Störungen des Phosphathaushalts
Journal für Klinische Endokrinologie und Stoffwechsel (2019)