Abstract
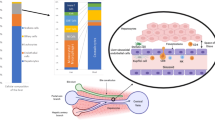
Receiving both portal vein blood and arterial blood, the liver is an important and critical component in the defense against blood-borne infection. To accomplish this role, the liver contains numerous innate and adaptive immune cells that specialize in detection and capture of pathogens from the blood. Further, these immune cells participate in coordinated immune responses leading to pathogen clearance, leukocyte recruitment and antigen presentation to lymphocytes within the vasculature. Finally, this role in host defense must be tightly regulated to ensure that inappropriate immune responses are not raised against nonpathogenic exogenous blood-borne molecules, such as those derived from food. It is this balance between activation and tolerance that characterizes the liver as a frontline immunological organ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ebe, Y. et al. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol. Int. 49, 519–532 (1999).
Lee, W.Y. et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 11, 295–302 (2010).
Helmy, K.Y. et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124, 915–927 (2006).This manuscript describes for the first time a new family of complement receptors, expressed predominately by macrophages, that are critical for capture of pathogens under flow conditions.
Sheth, K. & Bankey, P. The liver as an immune organ. Curr. Opin. Crit. Care 7, 99–104 (2001).
Crispe, I.N. Liver antigen-presenting cells. J. Hepatol. 54, 357–365 (2011).
Berg, R.D. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 3, 149–154 (1995).
Son, G., Kremer, M. & Hines, I.N. Contribution of gut bacteria to liver pathobiology. Gastroenterol. Res. Pract. 2010, 453563 (2010).
Lumsden, A.B., Henderson, J.M. & Kutner, M.H. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology 8, 232–236 (1988).
Paulos, C.M. et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 117, 2197–2204 (2007).
Oda, M., Yokomori, H. & Han, J.Y. Regulatory mechanisms of hepatic microcirculation. Clin. Hemorheol. Microcirc. 29, 167–182 (2003).
Racanelli, V. & Rehermann, B. The liver as an immunological organ. Hepatology 43, S54–S62 (2006).
Wisse, E. et al. Structure and function of sinusoidal lining cells in the liver. Toxicol. Pathol. 24, 100–111 (1996).This work, using electron microscopy, beautifully illustrates the anatomical features, fine structures and cell-cell interactions present in the liver microvasculature.
Wisse, E., De Zanger, R.B., Charels, K., Van Der, S.P. & McCuskey, R.S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 5, 683–692 (1985).
Kempka, G. & Kolb-Bachofen, V. Binding, uptake, and transcytosis of ligands for mannose-specific receptors in rat liver: an electron microscopic study. Exp. Cell Res. 176, 38–48 (1988).
Warren, A. et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 44, 1182–1190 (2006).
Wu, J. et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology 129, 363–374 (2010).
Knolle, P.A. & Limmer, A. Control of immune responses by savenger liver endothelial cells. Swiss Med. Wkly. 133, 501–506 (2003).
Knolle, P.A. et al. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin. Exp. Immunol. 114, 427–433 (1998).
Lohse, A.W. et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology 110, 1175–1181 (1996).
Steffan, A.M., Gendrault, J.L., McCuskey, R.S., McCuskey, P.A. & Kirn, A. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology 6, 830–836 (1986).
Sorensen, K.K. et al. The scavenger endothelial cell: a new player in homeostasis and immunity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R1217–R1230 (2012).
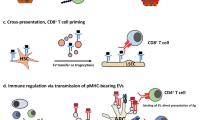
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6, 1348–1354 (2000).Using both in vitro and in vivo techniques, this work demonstrates the ability of LSECs to take up and cross-present antigens to T cells in the liver. This work also shows how this cross-presentation can result in rapid and efficient T cell tolerance in the liver.
Berg, M. et al. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur. J. Immunol. 36, 2960–2970 (2006).
Limmer, A. et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur. J. Immunol. 35, 2970–2981 (2005).
Tavassoli, M., Kishimoto, T. & Kataoka, M. Liver endothelium mediates the hepatocyte's uptake of ceruloplasmin. J. Cell Biol. 102, 1298–1303 (1986).
Diehl, L. et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 47, 296–305 (2008).
Knolle, P.A. et al. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J. Immunol. 162, 1401–1407 (1999).
Knolle, P. et al. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J. Hepatol. 22, 226–229 (1995).
Schildberg, F.A. et al. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur. J. Immunol. 38, 957–967 (2008).
Seki, E. & Brenner, D.A. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 48, 322–335 (2008).
Zhang, X. et al. Lipopolysaccharide-induced innate immune responses in primary hepatocytes downregulates woodchuck hepatitis virus replication via interferon-independent pathways. Cell. Microbiol. 11, 1624–1637 (2009).
Franco, A. et al. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology 8, 449–454 (1988).
Chen, M., Tabaczewski, P., Truscott, S.M., Van Kaer, L. & Stroynowski, I. Hepatocytes express abundant surface class I MHC and efficiently use transporter associated with antigen processing, tapasin, and low molecular weight polypeptide proteasome subunit components of antigen processing and presentation pathway. J. Immunol. 175, 1047–1055 (2005).
Wahl, C., Bochtler, P., Chen, L., Schirmbeck, R. & Reimann, J. B7–H1 on hepatocytes facilitates priming of specific CD8 T cells but limits the specific recall of primed responses. Gastroenterology 135, 980–988 (2008).
Balam, S., Romero, J.F., Bongfen, S.E., Guillaume, P. & Corradin, G. CSP–a model for in vivo presentation of Plasmodium berghei sporozoite antigens by hepatocytes. PLoS ONE 7, e51875 (2012).
Qian, S. et al. Hepatocyte-induced apoptosis of activated T cells, a mechanism of liver transplant tolerance, is related to the expression of ICAM-1 and hepatic lectin. Transplant. Proc. 33, 226 (2001).
Bertolino, P., McCaughan, G.W. & Bowen, D.G. Role of primary intrahepatic T-cell activation in the 'liver tolerance effect'. Immunol. Cell Biol. 80, 84–92 (2002).
Bertolino, P., Bowen, D.G., McCaughan, G.W. & Fazekas de St, G.B. Antigen-specific primary activation of CD8+ T cells within the liver. J. Immunol. 166, 5430–5438 (2001).
Bode, J.G., Albrecht, U., Haussinger, D., Heinrich, P.C. & Schaper, F. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur. J. Cell Biol. 91, 496–505 (2012).
Nemeth, E., Baird, A.W. & O'Farrelly, C. Microanatomy of the liver immune system. Semin. Immunopathol. 31, 333–343 (2009).
Gao, B., Jeong, W.I. & Tian, Z. Liver: an organ with predominant innate immunity. Hepatology 47, 729–736 (2008).
Parker, G.A. & Picut, C.A. Immune functioning in non lymphoid organs: the liver. Toxicol. Pathol. 40, 237–247 (2012).
Pannen, B.H. & Robotham, J.L. The acute-phase response. New Horiz. 3, 183–197 (1995).
Sarma, J.V. & Ward, P.A. The complement system. Cell Tissue Res. 343, 227–235 (2011).
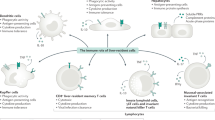
Bilzer, M., Roggel, F. & Gerbes, A.L. Role of Kupffer cells in host defense and liver disease. Liver Int. 26, 1175–1186 (2006).
Gale, R.P., Sparkes, R.S. & Golde, D.W. Bone marrow origin of hepatic macrophages (Kupffer cells) in humans. Science 201, 937–938 (1978).
Taniguchi, H., Toyoshima, T., Fukao, K. & Nakauchi, H. Presence of hematopoietic stem cells in the adult liver. Nat. Med. 2, 198–203 (1996).
Bouwens, L., Baekeland, M., De Zanger, R. & Wisse, E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology 6, 718–722 (1986).
Parker, G.A. & Picut, C.A. Liver immunobiology. Toxicol. Pathol. 33, 52–62 (2005).
Gorgani, N.N. et al. Complement receptor of the Ig superfamily enhances complement-mediated phagocytosis in a subpopulation of tissue resident macrophages. J. Immunol. 181, 7902–7908 (2008).
Gregory, S.H., Sagnimeni, A.J. & Wing, E.J. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 157, 2514–2520 (1996).This work describes for the first time a coordinated response between KCs and neutrophils, whereby the KC is critical for pathogen capture and the neutrophil is critical for pathogen killing. Though KCs could capture the bacteria, they could not fully internalize and kill the pathogen. To compensate for this, neutrophils were recruited to these bound bacteria and were responsible for pathogen killing.
You, Q., Cheng, L., Kedl, R.M. & Ju, C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology 48, 978–990 (2008).
Huang, L.R. et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8 T cells and successful immunotherapy against chronic viral liver infection. Nat. Immunol. 14, 574–583 (2013).Using CpG DNA, this study demonstrated a mechanism for overcoming T cell tolerance in the liver. Using a variety of techniques, including intravital microscopy, activated T cells were shown to form follicle-like structures in the liver, supporting proliferation and licensing of these cells as cytotoxic effector cells leading to the clearance of chronic viral infection from the liver.
Shi, J., Gilbert, G.E., Kokubo, Y. & Ohashi, T. Role of the liver in regulating numbers of circulating neutrophils. Blood 98, 1226–1230 (2001).
Shi, J., Fujieda, H., Kokubo, Y. & Wake, K. Apoptosis of neutrophils and their elimination by Kupffer cells in rat liver. Hepatology 24, 1256–1263 (1996).
Fadok, V.A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101, 890–898 (1998).
Grozovsky, R., Hoffmeister, K.M. & Falet, H. Novel clearance mechanisms of platelets. Curr. Opin. Hematol. 17, 585–589 (2010).
Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 21, 311–335 (2001).
Winau, F., Quack, C., Darmoise, A. & Kaufmann, S.H. Starring stellate cells in liver immunology. Curr. Opin. Immunol. 20, 68–74 (2008).
Bomble, M., Tacke, F., Rink, L., Kovalenko, E. & Weiskirchen, R. Analysis of antigen-presenting functionality of cultured rat hepatic stellate cells and transdifferentiated myofibroblasts. Biochem. Biophys. Res. Commun. 396, 342–347 (2010).
Winau, F. et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 26, 117–129 (2007).
Vinas, O. et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology 38, 919–929 (2003).
Muhanna, N., Horani, A., Doron, S. & Safadi, R. Lymphocyte-hepatic stellate cell proximity suggests a direct interaction. Clin. Exp. Immunol. 148, 338–347 (2007).
Yu, M.C. et al. Inhibition of T-cell responses by hepatic stellate cells via B7–H1-mediated T-cell apoptosis in mice. Hepatology 40, 1312–1321 (2004).
Ichikawa, S., Mucida, D., Tyznik, A.J., Kronenberg, M. & Cheroutre, H. Hepatic stellate cells function as regulatory bystanders. J. Immunol. 186, 5549–5555 (2011).
Chang, J. et al. Activated hepatic stellate cells mediate the differentiation of macrophages. Hepatol. Res. 43, 658–669 (2013).
Sato, T., Yamamoto, H., Sasaki, C. & Wake, K. Maturation of rat dendritic cells during intrahepatic translocation evaluated using monoclonal antibodies and electron microscopy. Cell Tissue Res. 294, 503–514 (1998).
Kudo, S., Matsuno, K., Ezaki, T. & Ogawa, M. A novel migration pathway for rat dendritic cells from the blood: hepatic sinusoids-lymph translocation. J. Exp. Med. 185, 777–784 (1997).
Matsuno, K., Ezaki, T., Kudo, S. & Uehara, Y. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph. J. Exp. Med. 183, 1865–1878 (1996).
Goddard, S., Youster, J., Morgan, E. & Adams, D.H. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am. J. Pathol. 164, 511–519 (2004).
Tokita, D. et al. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J. Hepatol. 49, 1008–1018 (2008).
Pillarisetty, V.G., Shah, A.B., Miller, G., Bleier, J.I. & DeMatteo, R.P. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J. Immunol. 172, 1009–1017 (2004).
Gao, X. et al. CD8+ DC, but not CD8− DC, isolated from BCG-infected mice reduces pathological reactions induced by mycobacterial challenge infection. PLoS ONE 5, e9281 (2010).
Yu, B. et al. Two immunogenic passenger dendritic cell subsets in the rat liver have distinct trafficking patterns and radiosensitivities. Hepatology 56, 1532–1545 (2012).
Pillarisetty, V.G., Katz, S.C., Bleier, J.I., Shah, A.B. & DeMatteo, R.P. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J. Immunol. 174, 2612–2618 (2005).This work is the first description and characterization of NK-DCs.
Chen, L. et al. Natural killer dendritic cells are an intermediate of developing dendritic cells. J. Leukoc. Biol. 81, 1422–1433 (2007).
Chaudhry, U.I. et al. Combined stimulation with interleukin-18 and CpG induces murine natural killer dendritic cells to produce IFN-gamma and inhibit tumor growth. Cancer Res. 66, 10497–10504 (2006).
Sana, G. et al. Adult human hepatocytes promote CD4+ T cell hyporesponsiveness via interleukin-10 producing allogeneic dendritic cells. Cell Transplant. doi:10.3727/096368913X666421 (12 April 2013).
Bamboat, Z.M. et al. Human liver dendritic cells promote T cell hyporesponsiveness. J. Immunol. 182, 1901–1911 (2009).
Kingham, T.P. et al. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology 45, 445–454 (2007).
Doherty, D.G. & O'Farrelly, C. Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 174, 5–20 (2000).
Crispe, I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Notas, G., Kisseleva, T. & Brenner, D. NK and NKT cells in liver injury and fibrosis. Clin. Immunol. 130, 16–26 (2009).
Abo, T., Kawamura, T. & Watanabe, H. Physiological responses of extrathymic T cells in the liver. Immunol. Rev. 174, 135–149 (2000).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
Seki, S., Abo, T., Ohteki, T., Sugiura, K. & Kumagai, K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J. Immunol. 147, 1214–1221 (1991).
Iiai, T. et al. Ontogeny and development of extrathymic T cells in mouse liver. Immunology 77, 556–563 (1992).
Watanabe, H. et al. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J. Immunol. 155, 2972–2983 (1995).
Brennan, P.J., Brigl, M. & Brenner, M.B. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13, 101–117 (2013).
Matsuda, J.L., Mallevaey, T., Scott-Browne, J. & Gapin, L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr. Opin. Immunol. 20, 358–368 (2008).
Wong, C.H., Jenne, C.N., Lee, W.Y., Leger, C. & Kubes, P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105 (2011).This work demonstrated for the first time the ability of NKT cells to activate in response to distal injury. This activation was mediated by neurotransmitters and resulted in the production of an anti-inflammatory, immunosuppressed state after ischemic stroke in the brain, rendering the animal susceptible to increased bacterial infection.
Stetson, D.B. et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076 (2003).
Geissmann, F. et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3, e113 (2005).This study provides the first description of a 'patrolling' lymphocyte in the liver microvasculature. Through the application of intravital microscopy, NKT cells were observed to reside in and actively crawl through the liver sinusoids, searching for pathogens.
Campos, R.A. et al. Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J. Exp. Med. 198, 1785–1796 (2003).
Campos, R.A. et al. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J. Immunol. 177, 3686–3694 (2006).
Wong, C.H., Jenne, C.N., Petri, B., Chrobok, N.L. & Kubes, P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat. Immunol. 14, 785–792 (2013).
Jenne, C.N., Urrutia, R. & Kubes, P. Platelets: bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 35, 254–261 (2013).
Smedsrod, B., Pertoft, H., Gustafson, S. & Laurent, T.C. Scavenger functions of the liver endothelial cell. Biochem. J. 266, 313–327 (1990).
Schafer, G., Guler, R., Murray, G., Brombacher, F. & Brown, G.D. The role of scavenger receptor B1 in infection with Mycobacterium tuberculosis in a murine model. PLoS ONE 4, e8448 (2009).
Eyre, N.S., Drummer, H.E. & Beard, M.R. The SR-BI partner PDZK1 facilitates hepatitis C virus entry. PLoS Pathog. 6, e1001130 (2010).
Dreux, M. et al. Receptor complementation and mutagenesis reveal SR-BI as an essential HCV entry factor and functionally imply its intra- and extra-cellular domains. PLoS Pathog. 5, e1000310 (2009).
Magnusson, S. & Berg, T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochem. J. 257, 651–656 (1989).
Stahl, P.D. & Ezekowitz, R.A. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10, 50–55 (1998).
Kerrigan, A.M. & Brown, G.D. C-type lectins and phagocytosis. Immunobiology 214, 562–575 (2009).
McDonald, B. et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J. Exp. Med. 205, 915–927 (2008).
Jenne, C.N., Wong, C.H., Petri, B. & Kubes, P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS ONE 6, e25109 (2011).
Jenne, C.N. et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 13, 169–180 (2013).
Gitlin, L. et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103, 8459–8464 (2006).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Tang, E.D. & Wang, C.Y. MAVS self-association mediates antiviral innate immune signaling. J. Virol. 83, 3420–3428 (2009).
Takeuchi, O. & Akira, S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 20, 17–22 (2008).
Eksioglu, E.A. et al. Characterization of HCV interactions with Toll-like receptors and RIG-I in liver cells. PLoS ONE 6, e21186 (2011).
Scott, M.J., Chen, C., Sun, Q. & Billiar, T.R. Hepatocytes express functional NOD1 and NOD2 receptors: a role for NOD1 in hepatocyte CC and CXC chemokine production. J. Hepatol. 53, 693–701 (2010).
Monteiro, R.C. & Van De Winkel, J.G. IgA Fc receptors. Annu. Rev. Immunol. 21, 177–204 (2003).
Ganesan, L.P. et al. FcgammaRIIb on liver sinusoidal endothelium clears small immune complexes. J. Immunol. 189, 4981–4988 (2012).
Bhatia, A., Blades, S., Cambridge, G. & Edwards, J.C. Differential distribution of Fc gamma RIIIa in normal human tissues and co-localization with DAF and fibrillin-1: implications for immunological microenvironments. Immunology 94, 56–63 (1998).
Lovdal, T. & Berg, T. Transcription of Fc(gamma) receptors in different rat liver cells. Cell Biol. Int. 25, 821–824 (2001).
Ravetch, J.V. & Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 19, 275–290 (2001).
Skogh, T., Blomhoff, R., Eskild, W. & Berg, T. Hepatic uptake of circulating IgG immune complexes. Immunology 55, 585–594 (1985).
Kosugi, I., Muro, H., Shirasawa, H. & Ito, I. Endocytosis of soluble IgG immune complex and its transport to lysosomes in hepatic sinusoidal endothelial cells. J. Hepatol. 16, 106–114 (1992).
He, J.Q. et al. CRIg mediates early Kupffer cell responses to adenovirus. J. Leukoc. Biol. 93, 301–306 (2013).
Menezes, G.B. et al. Selective down-regulation of neutrophil Mac-1 in endotoxemic hepatic microcirculation via IL-10. J. Immunol. 183, 7557–7568 (2009).
McDonald, B., Urrutia, R., Yipp, B.G., Jenne, C.N. & Kubes, P. Intravascular Neutrophil Extracellular Traps Capture Bacteria from the Bloodstream during Sepsis. Cell Host Microbe 12, 324–333 (2012).
Bonder, C.S. et al. Rules of recruitment for Th1 and Th2 lymphocytes in inflamed liver: a role for alpha-4 integrin and vascular adhesion protein-1. Immunity 23, 153–163 (2005).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Imaeda, A.B. et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 119, 305–314 (2009).
Marques, P.E. et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56, 1971–1982 (2012).
Wong, J. et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J. Clin. Invest. 99, 2782–2790 (1997).
Aspinall, A.I. et al. CX(3)CR1 and vascular adhesion protein-1-dependent recruitment of CD16(+) monocytes across human liver sinusoidal endothelium. Hepatology 51, 2030–2039 (2010).
Thomas, S.Y. et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J. Exp. Med. 208, 1179–1188 (2011).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).This is the first description of NETs, a bacterial killing mechanism whereby neutrophil nuclear DNA decondenses and is ejected from the cell, forming a diffuse, sticky web decorated with numerous nuclear and granule proteins.
Acknowledgements
We are grateful to W.-Y. Lee and C. Wong (Monash University, Australia) for providing the intravital microscopy images and movie used in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rapid target capture by Kupffer cells under flow conditions.
Intravital microscopy of the liver of a C57BL/6 mouse. After intravenous injection of inert, fluorescent microspheres (green) F4/80-labeled Kupffer cells (blue) are seen to capture the particles from the circulation under shear conditions. (MOV 1803 kb)
Rights and permissions
About this article
Cite this article
Jenne, C., Kubes, P. Immune surveillance by the liver. Nat Immunol 14, 996–1006 (2013). https://doi.org/10.1038/ni.2691
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2691
This article is cited by
-
Progress of immune checkpoint inhibitors therapy for non-small cell lung cancer with liver metastases
British Journal of Cancer (2024)
-
Cryo-EM structures of type IV pili complexed with nanobodies reveal immune escape mechanisms
Nature Communications (2024)
-
The significance of m6A RNA methylation regulators in diagnosis and subtype classification of HBV-related hepatocellular carcinoma
Human Cell (2024)
-
Acute-on-chronic liver failure: far to go—a review
Critical Care (2023)
-
Novel antigens for targeted radioimmunotherapy in hepatocellular carcinoma
Molecular and Cellular Biochemistry (2023)