Abstract
Introduction
Breast cancer (BC) is the most common type of cancer diagnosed among women worldwide with an estimated 2.3 million new cases every year. Almost two-thirds of all patients with BC have estrogen receptor-positive (ER+) tumors. In this review, the clinical evidence of exemestane in different treatment settings in ER+ BC is presented and summarized.
Search strategy
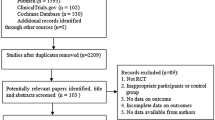
A search strategy with the keywords “breast cancer [MeSH Terms]” AND “exemestane [Title/Abstract]” was devised and a search was performed in PubMed.
Results
The efficacy of exemestane in different treatment settings has been established by numerous clinical studies. Exemestane is recommended as an adjuvant treatment in postmenopausal women previously treated with tamoxifen in trials comparing 5 years of tamoxifen with 2–3 years of tamoxifen combined with 2–3 years of exemestane, which proved that treatment with exemestane provided better survival outcomes. Similarly, exemestane could be considered as a safe treatment option for neoadjuvant treatment, prevention of chemotherapy, and treatment of advanced BC either alone or in combination with other targeted therapy drugs in both pre- and postmenopausal women.
Conclusion
Exemestane could be considered as a reasonable therapeutic option in the treatment of ER+ BC at any stage in pre- and postmenopausal women.

Similar content being viewed by others
References
Lumachi F, Santeufemia DA, Basso SM. Current medical treatment of estrogen receptor-positive breast cancer. World J Biol Chem. 2015;6:231–9.
Puhalla S, Bhattacharya S, Davidson NE. Hormonal therapy in breast cancer: A model disease for the personalization of cancer care. Mol Oncol [Internet]. 2012 [cited 2021 Apr 26];6:222–36. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5528370/.
Zucchini G, Geuna E, Milani A, Aversa C, Martinello R, Montemurro F. Clinical utility of exemestane in the treatment of breast cancer. IJWH [Internet]. Dove Press; 2015 [cited 2021 Apr 26];7:551–63. https://www.dovepress.com/clinical-utility-of-exemestane-in-the-treatment-ofnbspbreast-cancernbs-peer-reviewed-fulltext-article-IJWH.
Pistelli M, Mora AD, Ballatore Z, Berardi R. Aromatase inhibitors in premenopausal women with breast cancer: the state of the art and future prospects. Curr Oncol [Internet]. 2018 [cited 2021 Apr 26];25:e168–75. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5927796/.
Smith IE, Dowsett M, Yap Y-S, Walsh G, Lønning PE, Santen RJ, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol. 2006;24:2444–7.
Choueiri TK, Alemany CA, Abou-Jawde RM, Budd GT. Role of aromatase inhibitors in the treatment of breast cancer. Clin Ther. 2004;26:1199–214.
Brueggemeier RW, Hackett JC, Diaz-Cruz ES. Aromatase inhibitors in the treatment of breast cancer. Endocr Rev. 2005;26:331–45.
Brueggemeier RW. Update on the use of aromatase inhibitors in breast cancer. Expert Opin Pharmacother. 2006;7:1919–30.
Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–42.
Dutta U, Pant K. Aromatase inhibitors: past, present and future in breast cancer therapy. Med Oncol. 2008;25:113–24.
Howell A, Howell SJ, Clarke R, Anderson E. Where do selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) now fit into breast cancer treatment algorithms? J Steroid Biochem Mol Biol. 2001;79:227–37.
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–2.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–71.
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92.
Evans TR, Di Salle E, Ornati G, Lassus M, Benedetti MS, Pianezzola E, et al. Phase I and endocrine study of exemestane (FCE 24304), a new aromatase inhibitor, in postmenopausal women. Cancer Res. 1992;52:5933–9.
Judith MB, Lucy SK, Robert EC, John FF, Alan SC, Stephen EJ, et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol [Internet]. 2011 [cited 2021 May 8];30:709–17. https://europepmc.org/article/med/22042946.
Morden JP, Alvarez I, Bertelli G, Coates AS, Coleman R, Fallowfield L, et al. Long term follow-up of the Intergroup Exemestane Study (IES). J Clin Oncol [Internet]. 2017 [cited 2021 May 6];35:2507–14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6175047/.
Mamounas EP, Jeong J-H, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–71.
Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Láng I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–18.
Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–37.
Bernhard J, Luo W, Ribi K, Colleoni M, Burstein HJ, Tondini C, et al. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16:848–58.
Pagani O, Francis PA, Fleming GF, Walley BA, Viale G, Colleoni M, et al. Absolute Improvements in Freedom From Distant Recurrence to Tailor Adjuvant Endocrine Therapies for Premenopausal Women: Results From TEXT and SOFT. J Clin Oncol [Internet]. 2020 [cited 2021 Jun 30];38:1293–303. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7164485/.
Kittaneh M, Glück S. Exemestane in the adjuvant treatment of breast cancer in postmenopausal women. Breast Cancer (Auckl) [Internet]. 2011 [cited 2021 Apr 28];5:209–26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3201097/.
Litton JK, Bevers TB, Arun BK. Exemestane in the prevention setting. Ther Adv Med Oncol. 2012;4:107–12.
van de Velde CJH, Rea D, Seynaeve C, Putter H, Hasenburg A, Vannetzel J-M, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377:321–31.
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18.
De Placido S, Gallo C, De Laurentiis M, Bisagni G, Arpino G, Sarobba MG, et al. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet Oncol. 2018;19:474–85.
Ellis MJ, Babiera G, Unzeitig GW, Marcom PK, Guenther JM, Deshryver FK, et al. ACOSOG Z1031: a randomized phase II trial comparing exemestane, letrozole, and anastrozole in postmenopausal women with clinical stage II/III estrogen receptor-positive breast cancer. JCO [Internet]. Wolters Kluwer; 2010 [cited 2021 May 5];28:LBA513. https://doi.org/10.1200/jco.2010.28.18_suppl.lba513.
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol [Internet]. 2011 [cited 2021 May 5];29:2342–9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3107749/.
Barnadas A, Gil M, González S, Tusquets I, Muñoz M, Arcusa A, et al. Exemestane as primary treatment of oestrogen receptor-positive breast cancer in postmenopausal women: a phase II trial. Br J Cancer [Internet]. Nature Publishing Group; 2009 [cited 2021 May 5];100:442–9. https://www.nature.com/articles/6604868.
Hojo T, Kinoshita T, Imoto S, Shimizu C, Isaka H, Ito H, et al. Use of the neo-adjuvant exemestane in post-menopausal estrogen receptor-positive breast cancer: a randomized phase II trial (PTEX46) to investigate the optimal duration of preoperative endocrine therapy. The Breast [Internet]. 2013 [cited 2021 May 5];22:263–7. https://www.sciencedirect.com/science/article/pii/S0960977613000568.
Sato N, Masuda N, Morimoto T, Ueno T, Kanbayashi C, Kaneko K, et al. Neoadjuvant exemestane or exemestane plus docetaxel and cyclophosphamide tailored by clinicopathological response to 12 weeks’ exemestane exposure in patients with estrogen receptor-positive breast cancer: A multicenter, open-label, phase II study. Cancer Med. 2019;8:5468–81.
Decensi A, Dunn BK, Puntoni M, Gennari A, Ford LG. Exemestane for breast cancer prevention: a critical shift? Cancer Discov. 2012;2:25–40.
Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91.
Zhang Y, Simondsen K, Kolesar JM. Exemestane for primary prevention of breast cancer in postmenopausal women. Am J Health Syst Pharm. 2012;69:1384–8.
Gatti-Mays ME, Venzon D, Galbo CE, Singer A, Reynolds J, Makariou E, et al. Exemestane use in postmenopausal women at high risk for invasive breast cancer: evaluating biomarkers of efficacy and safety. Cancer Prev Res (Phila). 2016;9:225–33.
Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:2942–62.
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui S, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:806–15.
Yardley DA, Ismail-Khan RR, Melichar B, Lichinitser M, Munster PN, Klein PM, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol. 2013;31:2128–35.
Park YH, Kim T-Y, Kim GM, Kang SY, Park IH, Kim JH, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;20:1750–9.
Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak AB, Yardley DA, et al. Triplet therapy (continuous ribociclib, everolimus, exemestane) in HR+/HER2− advanced breast cancer postprogression on a CDK4/6 inhibitor (TRINITI-1): efficacy, safety, and biomarker results. JCO Wolters Kluwer. 2019;37:1016–1016.
Xu H-B, Liu Y-J, Li L. Aromatase inhibitor versus tamoxifen in postmenopausal woman with advanced breast cancer: a literature-based meta-analysis. Clin Breast Cancer. 2011;11:246–51.
Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–90.
Llombart-Cussac A, Ruiz A, Antón A, Barnadas A, Antolín S, Alés-Martínez JE, et al. Exemestane versus anastrozole as front-line endocrine therapy in postmenopausal patients with hormone receptor-positive, advanced breast cancer: final results from the Spanish Breast Cancer Group 2001–03 phase 2 randomized trial. Cancer. 2012;118:241–7.
Lønning PE. Lack of complete cross-resistance between different aromatase inhibitors; a real finding in search for an explanation? Eur J Cancer. 2009;45:527–35.
Beresford M, Tumur I, Chakrabarti J, Barden J, Rao N, Makris A. A qualitative systematic review of the evidence base for non-cross-resistance between steroidal and non-steroidal aromatase inhibitors in metastatic breast cancer. Clin Oncol (R Coll Radiol). 2011;23:209–15.
Kim SH, Park IH, Lee H, Lee KS, Nam B-H, Ro J. Efficacy of exemestane after nonsteroidal aromatase inhibitor use in metastatic breast cancer patients. Asian Pac J Cancer Prev. 2012;13:979–83.
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70.
Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14:989–98.
Xie Y, Li Y, Zhang Y, Zhang S, Li W, Guan X, et al. Fulvestrant 500 mg versus exemestane in postmenopausal women with metastatic breast cancer resistant to adjuvant nonsteroidal aromatase inhibitors in clinical practice: a multicenter retrospective study. Clinical Breast Cancer [Internet]. 2019 [cited 2021 Jun 29];19:e452–8. https://linkinghub.elsevier.com/retrieve/pii/S1526820918308012.
Li Y, Xie Y, Gong C, Zhao Y, Zhang J, Zhang S, et al. Comparative treatment patterns and outcomes of fulvestrant versus everolimus plus exemestane for postmenopausal metastatic breast cancer resistant to aromatase inhibitors in real-world experience. Ther Clin Risk Manag [Internet]. 2020 [cited 2021 Jun 29];16:607–15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7335268/.
Yardley DA, Noguchi S, Pritchard KI, Burris HA, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–84.
Steger GG, Egle D, Bartsch R, Pfeiler G, Petru E, Greil R, et al. Efficacy and safety of everolimus plus exemestane in patients with HR+, HER2− advanced breast cancer progressing on/after prior endocrine therapy in routine clinical practice: Primary results from the non-interventional study, STEPAUT. Breast [Internet]. 2020 [cited 2021 Jun 29];50:64–70. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7375626/.
Maass N, Ostermann H, Possinger K, Klein P, Tesch H, Mühlenhoff L, et al. ACT-FASTER, a prospective cohort study exploring treatment patterns with fulvestrant and exemestane in postmenopausal patients with advanced hormone receptor-positive breast cancer under real-life conditions in Germany. Breast Care (Basel) [Internet]. 2019 [cited 2021 Jun 29];14:401–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6940445/.
Chen S. An “Omics” approach to determine the mechanisms of acquired aromatase inhibitor resistance. OMICS [Internet]. Mary Ann Liebert, Inc., publishers; 2011 [cited 2021 May 3];15:347–52. https://doi.org/10.1089/omi.2010.0097.
Masri S, Phung S, Wang X, Chen S. Molecular characterization of aromatase inhibitor-resistant, tamoxifen-resistant and LTEDaro cell lines. J Steroid Biochem Mol Biol. 2010;118:277–82.
Wang X, Masri S, Phung S, Chen S. The role of amphiregulin in exemestane-resistant breast cancer cells: evidence of an autocrine loop. Cancer Res. 2008;68:2259–65.
Mills JN, Rutkovsky AC, Giordano A. Mechanisms of resistance in estrogen receptor positive breast cancer: overcoming resistance to tamoxifen/aromatase inhibitors. Curr Opin Pharmacol [Internet]. 2018 [cited 2021 May 4];41:59–65. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6454890/.
Hole S, Pedersen AM, Lykkesfeldt AE, Yde CW. Aurora kinase A and B as new treatment targets in aromatase inhibitor-resistant breast cancer cells. Breast Cancer Res Treat [Internet]. 2015 [cited 2021 May 4];149:715–26. https://doi.org/10.1007/s10549-015-3284-8.
Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44.
Khatri R, Shah P, Guha R, Rassool FV, Tomkinson AE, Brodie A, et al. Retraction: aromatase inhibitor-mediated downregulation of INrf2 (Keap1) leads to increased Nrf2 and resistance in breast cancer. Mol Cancer Ther. 2018;17:2491.
Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47.
Amaral C, Lopes A, Varela CL, da Silva ET, Roleira FMF, Correia-da-Silva G, et al. Exemestane metabolites suppress growth of estrogen receptor-positive breast cancer cells by inducing apoptosis and autophagy: a comparative study with Exemestane. Int J Biochem Cell Biol. 2015;69:183–95.
Augusto TV, Correia-da-Silva G, Rodrigues CMP, Teixeira N, Amaral C. Acquired resistance to aromatase inhibitors: where we stand! Endocrine-Related Cancer [Internet]. Bioscientifica Ltd; 2018 [cited 2021 May 4];25:R283–301. https://erc.bioscientifica.com/view/journals/erc/25/5/ERC-17-0425.xml.
Sobral AF, Amaral C, Correia-da-Silva G, Teixeira N. Unravelling exemestane: from biology to clinical prospects. J Steroid Biochem Mol Biol. 2016;163:1–11.
Ma CX, Reinert T, Chmielewska I, Ellis MJ. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15:261–75.
Chen Z, Yuan Y-C, Wang Y, Liu Z, Chan HJ, Chen S. Down-regulation of programmed cell death 4 (PDCD4) is associated with aromatase inhibitor resistance and a poor prognosis in estrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2015;152:29–39.
Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–70.
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med [Internet]. Massachusetts Medical Society; 2004 [cited 2021 May 10];350:1081–92. https://doi.org/10.1056/NEJMoa040331.
Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299–309.
Kieback DG, Harbeck N, Bauer W, Hadji P, Weyer G, Menschik T, et al. Endometrial effects of exemestane compared to tamoxifen within the Tamoxifen Exemestane Adjuvant Multicenter (TEAM) trial: results of a prospective gynecological ultrasound substudy. Gynecol Oncol. 2010;119:500–5.
Jones SE, Seynaeve C, Hasenburg A, Rae D, Vannetzel J, Paridaens R, et al. Results of the first planned analysis of the TEAM (tamoxifen exemestane adjuvant multinational) prospective randomized phase III trial in hormone sensitive postmenopausal early breast cancer. Cancer Res [Internet]. American Association for Cancer Research; 2009 [cited 2021 May 10];69:15. https://cancerres.aacrjournals.org/content/69/2_Supplement/15.
Markopoulos C, Polychronis A, Zobolas V, Xepapadakis G, Papadiamantis J, Koukouras D, et al. The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: preliminary results of the TEAM Greek sub-study. Breast Cancer Res Treat. 2005;93:61–6.
Hadji P, Asmar L, van Nes JGH, Menschik T, Hasenburg A, Kuck J, et al. The effect of exemestane and tamoxifen on bone health within the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial: a meta-analysis of the US, German, Netherlands, and Belgium sub-studies. J Cancer Res Clin Oncol. 2011;137:1015–25.
Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28:1294–300.
Goss PE, Hershman DL, Cheung AM, Ingle JN, Khosla S, Stearns V, et al. Effects of adjuvant exemestane versus anastrozole on bone mineral density for women with early breast cancer (MA.27B): a companion analysis of a randomised controlled trial. Lancet Oncol. 2014;15:474–82.
Gnant M, Baselga J, Rugo HS, Noguchi S, Burris HA, Piccart M, et al. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J Natl Cancer Inst. 2013;105:654–63.
Lønning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. JCO [Internet]. Wolters Kluwer; 2005 [cited 2021 May 10];23:5126–37. https://doi.org/10.1200/JCO.2005.07.097.
Wang X, Zhu A, Wang J, Ma F, Liu J, Fan Y, et al. Steroidal aromatase inhibitors have a more favorable effect on lipid profiles than nonsteroidal aromatase inhibitors in postmenopausal women with early breast cancer: a prospective cohort study. Ther Adv Med Oncol [Internet]. 2020 [cited 2021 Jun 16];12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7252381/.
Saini KS, Twelves C. Determining lines of therapy in patients with solid cancers: a proposed new systematic and comprehensive framework. Br J Cancer [Internet]. 2021 [cited 2021 Aug 26];125:155–63. https://www.nature.com/articles/s41416-021-01319-8.
Derks MGM, Blok EJ, Seynaeve C, Nortier JWR, Kranenbarg EM-K, Liefers G-J, et al. Adjuvant tamoxifen and exemestane in women with postmenopausal early breast cancer (TEAM): 10-year follow-up of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1211–20.
Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—a randomized controlled phase III trial. J Clin Oncol. 2013;31:1398–404.
Goss PE, Hershman DL, Cheung AM, Ingle JN, Khosla S, Stearns V, et al. Effects of adjuvant exemestane versus anastrozole on bone mineral density for women with early breast cancer (MA.27B): a companion analysis of a randomised controlled trial. Lancet Oncol [Internet]. 2014 [cited 2021 May 7];15:474–82. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4352316/.
Alba E, Calvo L, Albanell J, De la Haba JR, Arcusa Lanza A, Chacon JI, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23:3069–74.
Semiglazov V, Kletsel A, Semiglazov V, Zhiltzova E, Ivanov V, Dashyan G, et al. Exemestane (E) vs tamoxifen (T) as neoadjuvant endocrine therapy for postmenopausal women with ER+ breast cancer (T2N1–2, T3N0–1, T4N0M0). JCO [Internet]. Wolters Kluwer; 2005 [cited 2021 May 5];23:530. https://doi.org/10.1200/jco.2005.23.16_suppl.530.
Lustberg MB, Povoski SP, Zhao W, Ziegler RM, Sugimoto Y, Ruppert AS, et al. Phase II trial of neoadjuvant exemestane in combination with celecoxib in postmenopausal women who have breast cancer. Clin Breast Cancer. 2011;11:221–7.
Cigler T, Richardson H, Yaffe MJ, Fabian CJ, Johnston D, Ingle JN, et al. A randomized, placebo-controlled trial (NCIC CTG MAP.2) examining the effects of exemestane on mammographic breast density, bone density, markers of bone metabolism and serum lipid levels in postmenopausal women. Breast Cancer Res Treat. 2011;126:453–61.
Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
Jerusalem G, de Boer RH, Hurvitz S, Yardley DA, Kovalenko E, Ejlertsen B, et al. Everolimus plus exemestane vs everolimus or capecitabine monotherapy for estrogen receptor-positive, HER2-negative advanced breast cancer: the BOLERO-6 randomized clinical trial. JAMA Oncol. 2018;4:1367–74.
Robertson JF, Ferrero J-M, Bourgeois H, Kennecke H, de Boer RH, Jacot W, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013;14:228–35.
Yamamoto Y, Ishikawa T, Hozumi Y, Ikeda M, Iwata H, Yamashita H, et al. Randomized controlled trial of toremifene 120 mg compared with exemestane 25 mg after prior treatment with a non-steroidal aromatase inhibitor in postmenopausal women with hormone receptor-positive metastatic breast cancer. BMC Cancer. 2013;13:239.
Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak A, Yardley DA, et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR+/HER2− advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin Cancer Res [Internet]. American Association for Cancer Research; 2021 [cited 2021 Jun 29]. https://clincancerres.aacrjournals.org/content/early/2021/06/18/1078-0432.CCR-20-2114.
Cook MM, Al Rabadi L, Kaempf AJ, Saraceni MM, Savin MA, Mitri ZI. Everolimus plus exemestane treatment in patients with metastatic hormone receptor‐positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist [Internet]. 2021 [cited 2021 Jun 29];26:101–6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7873317/.
Cazzaniga ME, Airoldi M, Arcangeli V, Artale S, Atzori F, Ballerio A, et al. Efficacy and safety of everolimus and exemestane in hormone-receptor positive (HR+) human-epidermal-growth-factor negative (HER2−) advanced breast cancer patients: new insights beyond clinical trials. The EVA Study Breast. 2017;35:115–21.
Acknowledgements
Funding
This study and the Rapid Service Fee were funded by Pfizer Medical Affairs, Shanghai, China.
Medical Writing, Editorial, and Other Assistance
We thank the Pfizer Medical Affairs team along with Dr Amit Bhat, Dr Kaushik and Dr Akhtar from Indegene Pvt Ltd for their valuable inputs and support; no financial compensation was given for their kind support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
All authors have contributed equally to the study.
Disclosures
Yongmei Wang, Fanbo Jing, and Haibo Wang have no conflict of interest to declare.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new study with human participants or animals performed by any of the authors.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to data confidentiality but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Jing, F. & Wang, H. Role of Exemestane in the Treatment of Estrogen-Receptor-Positive Breast Cancer: A Narrative Review of Recent Evidence. Adv Ther 39, 862–891 (2022). https://doi.org/10.1007/s12325-021-01924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01924-2