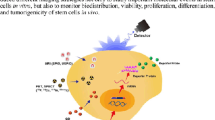
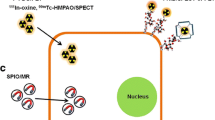
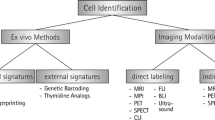
Abstract
Stem cell based-therapies are novel therapeutic strategies that hold key for developing new treatments for diseases conditions with very few or no cures. Although there has been an increase in the number of clinical trials involving stem cell-based therapies in the last few years, the long-term risks and benefits of these therapies are still unknown. Detailed in vivo studies are needed to monitor the fate of transplanted cells, including their distribution, differentiation, and longevity over time. Advancements in non-invasive cellular imaging techniques to track engrafted cells in real-time present a powerful tool for determining the efficacy of stem cell-based therapies. In this review, we describe the latest approaches to stem cell labeling and tracking using different imaging modalities.






Similar content being viewed by others
References
Jacobson, L. O., Simmons, E. L., & Bethard, W. F. (1950). Studies on hematopoietic recovery from radiation injury. The Journal of Clinical Investigation, 29(6), 825.
Simmons, E. L., Jacobson, L. O., Marks, E. K., & Gaston, E. O. (1959). Long-term survival of irradiated mice treated with homologous tissue suspensions. Nature, 183(4660), 556.
Jacobson, L. O., & Simmons, E. L. (1960). Comparison of the effects of isologous, homologous, and heterologous hematopoietic tissues on post-irradiation survival. Radiology, 75, 6–10.
Barnes, D. W., & Loutit, J. F. (1953). Protective effects of implants of splenic tissue. Proceedings of the Royal Society of Medicine, 46(4), 251–252.
Main, J. M., & Prehn, R. T. (1957). Fate of skin homografts in x-irradiated mice treated with homologous marrow. Journal of the National Cancer Institute, 19(6), 1053–1064.
Thomas, E. D., Lochte, H. L., Jr., Cannon, J. H., Sahler, O. D., & Ferrebee, J. W. (1959). Supralethal whole body irradiation and isologous marrow transplantation in man. The Journal of Clinical Investigation, 38, 1709–1716.
Hatzistergos, K. E., Blum, A., Ince, T., Grichnik, J. M., & Hare, J. M. (2011). What is the oncologic risk of stem cell treatment for heart disease? Circulation Research, 108(11), 1300–1303.
Rocha, V., Wagner, J. E., Jr., Sobocinski, K. A., et al. (2000). Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. The New England Journal of Medicine, 342(25), 1846–1854.
Nair, G., Tanahashi, Y., Low, H. P., Billings-Gagliardi, S., Schwartz, W. J., & Duong, T. Q. (2005). Myelination and long diffusion times alter diffusion-tensor-imaging contrast in myelin-deficient shiverer mice. NeuroImage, 28(1), 165–174.
Rickers, C., Gallegos, R., Seethamraju, R. T., et al. (2004). Applications of magnetic resonance imaging for cardiac stem cell therapy. Journal of Interventional Cardiology, 17(1), 37–46.
Wu, C., Zhu, J., Baeslack, J., et al. (2013). Longitudinal PET imaging for monitoring myelin repair in the spinal cord. Annals of Neurology. doi:10.1002/ana.23965.
Voura, E. B., Jaiswal, J. K., Mattoussi, H., & Simon, S. M. (2004). Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nature Medicine, 10(9), 993–998.
Lei, Y., Tang, H., Yao, L., Yu, R., Feng, M., & Zou, B. (2008). Applications of mesenchymal stem cells labeled with Tat peptide conjugated quantum dots to cell tracking in mouse body. Bioconjugate Chemistry, 19(2), 421–427.
Ohyabu, Y., Kaul, Z., Yoshioka, T., et al. (2009). Stable and nondisruptive in vitro/in vivo labeling of mesenchymal stem cells by internalizing quantum dots. Human Gene Therapy, 20(3), 217–224.
Sugiyama, T., Kuroda, S., Osanai, T., et al. (2011). Near-infrared fluorescence labeling allows noninvasive tracking of bone marrow stromal cells transplanted into rat infarct brain. Neurosurgery, 68(4), 1036–1047.
Rak-Raszewska, A., Marcello, M., Kenny, S., Edgar, D., See, V., & Murray, P. (2012). Quantum dots do not affect the behaviour of mouse embryonic stem cells and kidney stem cells and are suitable for short-term tracking. PLoS One, 7(3), e32650.
Li, K., Qin, W., Ding, D., et al. (2013). Photostable fluorescent organic dots with aggregation-induced emission (AIE dots) for noninvasive long-term cell tracing. Scientific Reports, 3, 1150.
Eisenblatter, M., Ehrchen, J., Varga, G., et al. (2009). In vivo optical imaging of cellular inflammatory response in granuloma formation using fluorescence-labeled macrophages. Journal of Nuclear Medicine, 50(10), 1676–1682.
Ruan, J., Song, H., Li, C., et al. (2012). DiR-labeled embryonic stem cells for targeted imaging of in vivo gastric cancer cells. Theranostics, 2(6), 618–628.
Shan, L. (2004). Near-infrared fluorescence 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR)-labeled macrophages for cell imaging. Molecular Imaging and Contrast Agent Database (MICAD) (Internet). Bethsda (MD): National Centre For Biotechnology Information (US); 2004–2013.
Frangioni, J. V. (2003). In vivo near-infrared fluorescence imaging. Current Opinion in Chemical Biology, 7(1), 626–634.
Sykova, E., & Jendelova, P. (2007). Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death and Differentiation, 14(7), 1336–1342.
Bulte, J. W., Hoekstra, Y., Kamman, R. L., et al. (2003). Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology, 228(2), 480–487.
Frank, J. A., Miller, B. R., Arbab, A. S., et al. (2003). Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology, 228(2), 480–487.
Hedlund, A., Ahren, M., Gustafsson, H., et al. (2011). Gd(2)O(3) nanoparticles in hematopoietic cells for MRI contrast enhancement. International Journal of Nanomedicine, 6, 3233–3240.
Agudelo, C. A., Tachibana, Y., Hurtado, A. F., Ose, T., Iida, H., & Yamaoka, T. (2012). The use of magnetic resonance cell tracking to monitor endothelial progenitor cells in a rat hindlimb ischemic model. Biomaterials, 33(8), 2439–2448.
Guenoun, J., Koning, G. A., Doeswijk, G., et al. (2012). Cationic Gd-DTPA liposomes for highly efficient labeling of mesenchymal stem cells and cell tracking with MRI. Cell Transplantation, 21(1), 191–205.
Modo, M., Beech, J. S., Meade, T. J., Williams, S. C., & Price, J. (2009). A chronic 1 year assessment of MRI contrast agent-labelled neural stem cell transplants in stroke. NeuroImage, 47(Suppl 2), T133–T142.
Rudelius, M., Daldrup-Link, H. E., Heinzmann, U., et al. (2003). Highly efficient paramagnetic labelling of embryonic and neuronal stem cells. European Journal of Nuclear Medicine and Molecular Imaging, 30(7), 1038–1044.
Bhorade, R., Weissleder, R., Nakakoshi, T., Moore, A., & Tung, C. H. (2000). Macrocyclic chelators with paramagnetic cations are internalized into mammalian cells via a HIV-tat derived membrane translocation peptide. Bioconjugate Chemistry, 11(3), 301–305.
Tseng, C. L., Shih, I. L., Stobinski, L., & Lin, F. H. (2010). Gadolinium hexanedione nanoparticles for stem cell labeling and tracking via magnetic resonance imaging. Biomaterials, 31(20), 5427–5435.
Ghaghada, K. B., Ravoori, M., Sabapathy, D., Bankson, J., Kundra, V., & Annapragada, A. (2009). New dual mode gadolinium nanoparticle contrast agent for magnetic resonance imaging. PLoS One, 4(10), e7628.
Klasson, A., Ahren, M., Hellqvist, E., et al. (2008). Positive MRI contrast enhancement in THP-1 cells with Gd2O3 nanoparticles. Contrast Media & Molecular Imaging, 3(3), 106–111.
Faucher, L., Tremblay, M., Lagueux, J., Gossuin, Y., & Fortin, M. A. (2012). Rapid synthesis of PEGylated ultrasmall gadolinium oxide nanoparticles for cell labeling and tracking with MRI. ACS Applied Materials & Interfaces, 4(9), 4506–4515.
Ward, K. M., Aletras, A. H., & Balaban, R. S. (2000). A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). Journal of Magnetic Resonance, 143(1), 79–87.
Aime, S., Carrera, C., Delli Castelli, D., Geninatti Crich, S., Terreno, E. (2005). Tunable imaging of cells labeled with MRI-PARACEST agents. Angewandte Chemie International Edition England, 44(12): 1813–1815.
Ferrauto, G., Castelli, D. D., Terreno, E., & Aime, S. (2013). In vivo MRI visualization of different cell populations labeled with PARACEST agents. Magnetic Resonance in Medicine, 69(6), 1703–1711.
Silva, A. C., & Bock, N. A. (2008). Manganese-enhanced MRI: an exceptional tool in translational neuroimaging. Schizophrenia Bulletin, 34(4), 595–604.
Gilad, A. A., Walczak, P., McMahon, M. T., et al. (2008). MR tracking of transplanted cells with "positive contrast" using manganese oxide nanoparticles. Magnetic Resonance in Medicine, 60(1), 1–7.
Kim, T., Momin, E., Choi, J., et al. (2011). Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. Journal of the American Chemical Society, 133(9), 2955–2961.
Josephson, L., Lewis, J., Jacobs, P., Hahn, P. F., & Stark, D. D. (1988). The effects of iron oxides on proton relaxivity. Magnetic Resonance Imaging, 6(6), 647–6453.
Shen, T., Weissleder, R., Papisov, M., Bogdanov, A., Jr., & Brady, T. J. (1993). Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magnetic Resonance in Medicine, 29(5), 599–604.
Jung, C. W. (1995). Surface properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magnetic Resonance Imaging, 13(5), 675–691.
Wagner, S., Schnorr, J., Pilgrimm, H., Hamm, B., & Taupitz, M. (2002). Monomer-coated very small superparamagnetic iron oxide particles as contrast medium for magnetic resonance imaging: preclinical in vivo characterization. Investigative Radiology, 37(4), 167–177.
Shapiro, E. M., Skrtic, S., Sharer, K., Hill, J. M., Dunbar, C. E., & Koretsky, A. P. (2004). MRI detection of single particles for cellular imaging. Proceedings of the National Academy of Sciences of the United States of America, 101(30), 10901–10906.
Hao, R., Xing, R., Xu, Z., Hou, Y., Gao, S., & Sun, S. (2010). Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Advanced Materials, 22(25), 2729–2742.
Rogers, W. J., Meyer, C. H., & Kramer, C. M. (2006). Technology insight: in vivo cell tracking by use of MRI. Nature clinical practice. Cardiovascular Medicine, 3(10), 554–562.
Norman, A. B., Thomas, S. R., Pratt, R. G., Lu, S. Y., & Norgren, R. B. (1992). Magnetic resonance imaging of neural transplants in rat brain using a superparamagnetic contrast agent. Brain Research, 594(2), 279–283.
Bulte, J. W., Brooks, R. A., Moskowitz, B. M., Bryant, L. H., Jr., & Frank, J. A. (1998). T1 and T2 relaxometry of monocrystalline iron oxide nanoparticles (MION-46L): theory and experiment. Academic Radiology, 5(Suppl 1), S137–S140.
Bulte, J. W., Zhang, S., van Gelderen, P., et al. (1999). Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proceedings of the National Academy of Sciences of the United States of America, 96(26), 15256–15261.
Bulte, J. W., Douglas, T., Witwer, B., et al. (2001). Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nature Biotechnology, 19(12), 1141–1147.
Karussis, D., Karageorgiou, C., Vaknin-Dembinsky, A. et al. (2010) Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Archives of Neurology, 67(10): 1187–1194.
Wang, Y., Wang, L., Che, Y., Li, Z., & Kong, D. (2011). Preparation and evaluation of magnetic nanoparticles for cell labeling. Journal of Nanoscience and Nanotechnology, 11(5), 3749–3756.
Nejadnik, H., Henning, T. D., Castaneda, R. T., et al. (2012). Somatic differentiation and MR imaging of magnetically labeled human embryonic stem cells. Cell Transplantation, 21(12), 2555–2567.
Richards, J. M., Shaw, C. A., Lang, N. N., et al. (2012). In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: feasibility and safety in humans. Circulation. Cardiovascular Imaging, 5(4), 509–517.
de Chickera, S. N., Snir, J., Willert, C., et al. (2011). Labelling dendritic cells with SPIO has implications for their subsequent in vivo migration as assessed with cellular MRI. Contrast Media & Molecular Imaging, 6(4), 314–327.
Dunning, M. D., Lakatos, A., Loizou, L., et al. (2004). Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. Journal of Neuroscience, 24(44), 9799–9810.
Huang, D. M., Hsiao, J. K., Chen, Y. C., et al. (2009). The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials, 30(22), 3645–3651.
Bulte, J. W. (2009). In vivo MRI cell tracking: clinical studies. American Journal of Roentgenology, 193(2), 314–325.
Ahrens, E. T., Feili-Hariri, M., Xu, H., Genove, G., & Morel, P. A. (2003). Receptor-mediated endocytosis of iron-oxide particles provides efficient labeling of dendritic cells for in vivo MR imaging. Magnetic Resonance in Medicine, 49(6), 1006–1013.
Modo, M., Hoehn, M., & Bulte, J. W. (2005). Cellular MR imaging. Molecular Imaging, 4(3), 143–164.
Walczak, P., Kedziorek, D. A., Gilad, A. A., Lin, S., & Bulte, J. W. (2005). Instant MR labeling of stem cells using magnetoelectroporation. Magnetic Resonance in Medicine, 54(4), 769–774.
Frank, J. A., Zywicke, H., Jordan, E. K., et al. (2002). Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Academic Radiology, 9(Suppl 2), S484–S487.
Kraitchman, D. L., & Bulte, J. W. (2008). Imaging of stem cells using MRI. Basic Research in Cardiology, 103(2), 105–113.
Bulte, J. W., Kraitchman, D. L., Mackay, A. M., & Pittenger, M. F. (2004). Chondrogenic differentiation of mesenchymal stem cells is inhibited after magnetic labeling with ferumoxides. Blood, 104(10), 3410–3412.
Walczak, P., Ruiz-Cabello, J., Kedziorek, D. A., et al. (2006). Magnetoelectroporation: improved labeling of neural stem cells and leukocytes for cellular magnetic resonance imaging using a single FDA-approved agent. Nanomedicine : Nanotechnology, Biology, and Medicine, 2(2), 89–94.
Terrovitis, J., Stuber, M., Youssef, A., et al. (2008). Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation, 117(12), 1555–1562.
Cromer Berman, S. M., Kshitiz, Wang, C. J., et al. (2013). Cell motility of neural stem cells is reduced after SPIO-labeling, which is mitigated after exocytosis. Magnetic Resonance in Medicine, 69(1), 255–262.
Barnett, B. P., Arepally, A., Karmarkar, P. V., et al. (2007). Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nature Medicine, 13(8), 986–991.
Shapiro, E. M., Sharer, K., Skrtic, S., & Koretsky, A. P. (2006). In vivo detection of single cells by MRI. Magnetic Resonance in Medicine, 55(2), 242–249.
Shapiro, E. M., Skrtic, S., & Koretsky, A. P. (2005). Sizing it up: cellular MRI using micron-sized iron oxide particles. Magnetic Resonance in Medicine, 53(2), 329–338.
Hinds, K. A., Hill, J. M., Shapiro, E. M., et al. (2003). Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood, 102(3), 867–872.
Shapiro, E. M., Medford-Davis, L. N., Fahmy, T. M., Dunbar, C. E., & Koretsky, A. P. (2007). Antibody-mediated cell labeling of peripheral T cells with micron-sized iron oxide particles (MPIOs) allows single cell detection by MRI. Contrast Media & Molecular Imaging, 2(3), 147–153.
Bernas, L. M., Foster, P. J., & Rutt, B. K. (2007). Magnetic resonance imaging of in vitro glioma cell invasion. Journal of Neurosurgery, 106(2), 306–313.
Foley, L. M., Hitchens, T. K., Ho, C., et al. (2009). Magnetic resonance imaging assessment of macrophage accumulation in mouse brain after experimental traumatic brain injury. Journal of Neurotrauma, 26(9), 1509–1519.
Sumner, J. P., Shapiro, E. M., Maric, D., Conroy, R., & Koretsky, A. P. (2009). In vivo labeling of adult neural progenitors for MRI with micron sized particles of iron oxide: quantification of labeled cell phenotype. NeuroImage, 44(3), 671–678.
Rohani, R., de Chickera, S. N., Willert, C., Chen, Y., Dekaban, G. A., & Foster, P. J. (2011). In vivo cellular MRI of dendritic cell migration using micrometer-sized iron oxide (MPIO) particles. Molecular Imaging and Biology, 13(4), 679–694.
Nkansah, M. K., Thakral, D., & Shapiro, E. M. (2011). Magnetic poly(lactide-co-glycolide) and cellulose particles for MRI-based cell tracking. Magnetic Resonance in Medicine, 65(6), 1776–1785.
Tang, K. S., & Shapiro, E. M. (2011). Enhanced magnetic cell labeling efficiency using -NH2 coated MPIOs. Magnetic Resonance in Medicine, 65(6), 1564–1569.
Ahrens, E. T., Flores, R., Xu, H., & Morel, P. A. (2005). In vivo imaging platform for tracking immunotherapeutic cells. Nature Biotechnology, 23(8), 983–987.
Partlow, K. C., Chen, J., Brant, J. A., et al. (2007). 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB Journal, 21(8), 1647–1654.
Ruiz-Cabello, J., Walczak, P., Kedziorek, D. A., et al. (2008). In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magnetic Resonance in Medicine, 60(6), 1506–1511.
Waiczies, H., Guenther, M., Skodowski, J., et al. (2013). Monitoring dendritic cell migration using 19F/1H Magnetic Resonance Imaging. Journal of Visulaized Experiments, 73, e50251.
Boehm-Sturm, P., Mengler, L., Wecker, S., Hoehn, M., & Kallur, T. (2011). In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One, 6(12), e29040.
Hitchens, T. K., Ye, Q., Eytan, D. F., Janjic, J. M., Ahrens, E. T., & Ho, C. (2011). 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magnetic Resonance in Medicine, 65(4), 1144–1153.
Bible, E., Dell'Acqua, F., Solanky, B., et al. (2012). Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials, 33(10), 2858–2871.
Waiczies, H., Lepore, S., Janitzek, N., et al. (2011). Perfluorocarbon particle size influences magnetic resonance signal and immunological properties of dendritic cells. PLoS One, 6(7), e21981.
Srinivas, M., Morel, P. A., Ernst, L. A., Laidlaw, D. H., & Ahrens, E. T. (2007). Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magnetic Resonance in Medicine, 58(4), 725–734.
Kadayakkara, D. K., Ranganathan, S., Young, W. B., & Ahrens, E. T. (2012). Assaying macrophage activity in a murine model of inflammatory bowel disease using fluorine-19 MRI. Laboratory Investigation, 92(4), 636–645.
Janjic, J. M., Srinivas, M., Kadayakkara, D. K., & Ahrens, E. T. (2008). Self-delivering nanoemulsions for dual fluorine-19 MRI and fluorescence detection. Journal of the American Chemical Society, 130(9), 2832–2841.
Verdijk, P., Scheenen, T. W., Lesterhuis, W. J., et al. (2007). Sensitivity of magnetic resonance imaging of dendritic cells for in vivo tracking of cellular cancer vaccines. International Journal of Cancer, 120(5), 978–984.
Srinivas, M., Heerschap, A., Ahrens, E. T., Figdor, C. G., & de Vries, I. J. (2010). (19)F MRI for quantitative in vivo cell tracking. Trends in Biotechnology, 28(7), 363–370.
Chan, K. W., Liu, G., Song, X., et al. (2013). MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nature Materials, 12(3), 268–275.
Massoud, T. F., & Gambhir, S. S. (2003). Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes & Development, 17(5), 545–580.
Adonai, N., Nguyen, K. N., Walsh, J., et al. (2002). Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proceedings of the National Academy of Sciences of the United States of America, 99(5), 3030–3035.
Zanzonico, P., Koehne, G., Gallardo, H. F., et al. (2006). [131I]FIAU labeling of genetically transduced, tumor-reactive lymphocytes: cell-level dosimetry and dose-dependent toxicity. European Journal of Nuclear Medicine and Molecular Imaging, 33(9), 988–997.
Bhargava, K. K., Gupta, R. K., Nichols, K. J., & Palestro, C. J. (2009). In vitro human leukocyte labeling with (64)Cu: an intraindividual comparison with (111)In-oxine and (18)F-FDG. Nuclear Medicine and Biology, 36(5), 545–549.
Brenner, W., Aicher, A., Eckey, T., et al. (2004). 111In-labeled CD34+ hematopoietic progenitor cells in a rat myocardial infarction model. Journal of Nuclear Medicine, 45(3), 512–518.
Rennen, H. J., Boerman, O. C., Oyen, W. J., & Corstens, F. H. (2001). Imaging infection/inflammation in the new millennium. European Journal of Nuclear Medicine, 28(2), 241–252.
Becker, W., & Meller, J. (2001). The role of nuclear medicine in infection and inflammation. The Lancet Infectious Diseases, 1(5), 326–333.
Jin, Y., Kong, H., Stodilka, R. Z., et al. (2005). Determining the minimum number of detectable cardiac-transplanted 111In-tropolone-labelled bone-marrow-derived mesenchymal stem cells by SPECT. Physics in Medicine and Biology, 50(19), 4445–4455.
Gholamrezanezhad, A., Mirpour, S., Bagheri, M., et al. (2011). In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nuclear Medicine and Biology, 38(7), 961–967.
Gholamrezanezhad, A., Mirpour, S., Ardekani, J. M., et al. (2009). Cytotoxicity of 111In-oxine on mesenchymal stem cells: a time-dependent adverse effect. Nuclear Medicine Communications, 30(3), 210–216.
Monteiro-Riviere, N. A., Inman, A. O., & Zhang, L. W. (2009). Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicology and Applied Pharmacology, 234(2), 222–235.
Barbash, I. M., Chouraqui, P., Baron, J., et al. (2003). Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation, 108(7), 863–868.
Kang, W. J., Kang, H. J., Kim, H. S., Chung, J. K., Lee, M. C., & Lee, D. S. (2006). Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. Journal of Nuclear Medicine, 47(8), 1295–1301.
Zhou, R., Thomas, D. H., Qiao, H., et al. (2005). In vivo detection of stem cells grafted in infarcted rat myocardium. Journal of Nuclear Medicine, 46(5), 816–822.
Swirski, F. K., Pittet, M. J., Kircher, M. F., et al. (2006). Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proceedings of the National Academy of Sciences of the United States of America, 103(27), 10340–10345.
Kim, J., Arifin, D. R., Muja, N., et al. (2011). Multifunctional capsule-in-capsules for immunoprotection and trimodal imaging. Angewandte Chemie International Edition England, 50(10), 2317–2321.
Arifin, D. R., Kedziorek, D. A., Fu, Y., et al. (2012). Microencapsulated cell tracking. NMR in Biomedicine. doi:10.1002/nbm.2894.
Link, T. W., Arifin, D. R., Long, C. M., et al. (2012). Use of magnetocapsules for in vivo visualization and enhanced survival of xenogeneic HepG2 cell transplants. Cell Medicine, 4(2), 77–784.
Arifin, D. R., Long, C. M., Gilad, A. A., et al. (2011). Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology, 260(3), 790–798.
Barnett, B. P., Kraitchman, D. L., Lauzon, C., et al. (2006). Radiopaque alginate microcapsules for X-ray visualization and immunoprotection of cellular therapeutics. Molecular Pharmaceutics, 3(5), 531–538.
Barnett, B. P., Ruiz-Cabello, J., Hota, P., et al. (2011). Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology, 258(1), 182–191.
Nam, S. Y., Ricles, L. M., Suggs, L. J., & Emelianov, S. Y. (2012). In vivo ultrasound and photoacoustic monitoring of mesenchymal stem cells labeled with gold nanotracers. PLoS One, 7(5), e37267.
Ricles, L. M., Nam, S. Y., Sokolov, K., Emelianov, S. Y., & Suggs, L. J. (2011). Function of mesenchymal stem cells following loading of gold nanotracers. International Journal of Nanomedicine, 6, 407–416.
Jokerst, J. V., Khademi, C., & Gambhir, S. S. (2013). Intracellular aggregation of multimodal silica nanoparticles for ultrasound-guided stem cell implantation. Science Translational Medicine, 5(177), 177ra35.
Cui, W., Tavri, S., Benchimol, M. J., et al. (2013). Neural progenitor cells labeling with microbubble contrast agent for ultrasound imaging in vivo. Biomaterials, 34(21), 4926–4935.
Forss-Petter, S., Danielson, P. E., Catsicas, S., et al. (1990). Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron, 5(2), 187–197.
Himes, S. R., & Shannon, M. F. (2000). Assays for transcriptional activity based on the luciferase reporter gene. Methods in Molecular Biology, 130, 165–174.
Contag, C. H., Jenkins, D., Contag, P. R., & Negrin, R. S. (2000). Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia, 2(1–2), 41–52.
Zhuo, L., Sun, B., Zhang, C. L., Fine, A., Chiu, S. Y., & Messing, A. (1997). Live astrocytes visualized by green fluorescent protein in transgenic mice. Developmental Biology, 187(1), 36–42.
Shaner, N. C., Campbell, R. E., Steinbach, P. A., Giepmans, B. N., Palmer, A. E., & Tsien, R. Y. (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnology, 22(12), 1567–1572.
Kremers, G. J., Gilbert, S. G., Cranfill, P. J., Davidson, M. W., & Piston, D. W. (2011). Fluorescent proteins at a glance. Journal of Cell Science, 124(2), 157–160.
Priddle, H., Grabowska, A., Morris, T., et al. (2009). Bioluminescence imaging of human embryonic stem cells transplanted in vivo in murine and chick models. Cloning and Stem Cells, 11(2), 259–267.
Love, Z., Wang, F., Dennis, J., et al. (2007). Imaging of mesenchymal stem cell transplant by bioluminescence and PET. Journal of Nuclear Medicine, 48(12), 2011–2020.
Tang, Y., Shah, K., Messerli, S. M., Snyder, E., Breakefield, X., & Weissleder, R. (2003). In vivo tracking of neural progenitor cell migration to glioblastomas. Human Gene Therapy, 14(13), 1247–1254.
Bai, X., Yan, Y., Coleman, M., et al. (2011). Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Molecular Imaging and Biology, 13(4), 633–645.
Janowski, M., Engels, C., Gorelik, M., et al. (2013). Survival of neural progenitors allografted into the CNS of immunocompetent recipients is highly dependent on transplantation site. Cell Transplant, doi: http://dx.doi.org/10.3727/096368912X661328.
Liang, Y., Agren, L., Lyczek, A., Walczak, P., & Bulte, J. W. (2013). Neural progenitor cell survival in mouse brain can be improved by co-transplantation of helper cells expressing bFGF under doxycycline control. Experimental Neurology, 247C, 73–79.
Maguire, K. K., Lim, L., Speedy, S., & Rando, T. A. (2013). Assessment of disease activity in muscular dystrophies by noninvasive imaging. The Journal of Clincal Investigation, 123(5), 2298–2305.
Liang, Y., Walczak, P., & Bulte, J. W. (2013). Comparison of red-shifted firefly luciferase Ppy RE9 and conventional Luc2 as bioluminescence imaging reporter genes for in vivo imaging of stem cells. Journal of Biomedical Optics, 17(1), 016004.
Ueda, I., Kamaya, H., & Eyring, H. (1976). Molecular mechanism of inhibition of firefly luminescence by local anesthetics. Proceedings of the National Academy of Sciences of the United States of America, 73(2), 481–485.
Inoue, Y., Kiryu, S., Izawa, K., Watanabe, M., Tojo, A., & Ohtomo, K. (2009). Comparison of subcutaneous and intraperitoneal injection of D-luciferin for in vivo bioluminescence imaging. European Journal of Nuclear Medicine and Molecular Imaging, 36(5), 771–779.
Keyaerts, M., Remory, I., Caveliers, V., et al. (2012). Inhibition of firefly luciferase by general anesthetics: effect on in vitro and in vivo bioluminescence imaging. PLoS One, 7(1), e30061.
Szarecka, A., Xu, Y., & Tang, P. (2007). Dynamics of firefly luciferase inhibition by general anesthetics: Gaussian and anisotropic network analyses. Biophysical Journal, 93(6), 1895–1905.
Keyaerts, M., Heneweer, C., Gainkam, L. O., et al. (2011). Plasma protein binding of luciferase substrates influences sensitivity and accuracy of bioluminescence imaging. Molecular Imaging and Biology, 13(1), 59–66.
Gilad, A. A., McMahon, M. T., Walczak, P., et al. (2007). Artificial reporter gene providing MRI contrast based on proton exchange. Nature Biotechnology, 25(2), 217–219.
Gilad, A. A., Ziv, K., McMahon, M. T., van Zijl, P. C., Neeman, M., & Bulte, J. W. (2008). MRI reporter genes. Journal of Nuclear Medicine, 49(12), 1905–1908.
Berman, S. C., Galpoththawela, C., Gilad, A. A., Bulte, J. W., & Walczak, P. (2011). Long-term MR cell tracking of neural stem cells grafted in immunocompetent versus immunodeficient mice reveals distinct differences in contrast between live and dead cells. Magnetic Resonance in Medicine, 65(2), 564–574.
Liu, G., Bulte, J. W., & Gilad, A. A. (2011). CEST MRI reporter genes. Methods in Molecular Biology, 711, 271–280.
Louie, A. Y., Huber, M. M., Ahrens, E. T., et al. (2000). In vivo visualization of gene expression using magnetic resonance imaging. Nature Biotechnology, 18(3), 321–325.
Cui, W., Liu, L., Kodibagkar, V. D., & Mason, R. P. (2010). S-Gal, a novel 1H MRI reporter for beta-galactosidase. Magnetic Resonance in Medicine, 64(1), 65–71.
Alfke, H., Stoppler, H., Nocken, F., et al. (2003). In vitro MR imaging of regulated gene expression. Radiology, 228(2), 488–492.
Gilad, A. A., Winnard, P. T., Jr., van Zijl, P. C., & Bulte, J. W. (2007). Developing MR reporter genes: promises and pitfalls. NMR in Biomedicine, 20(3), 275–290.
Weissleder, R., Moore, A., Mahmood, U., et al. (2000). In vivo magnetic resonance imaging of transgene expression. Nature Medicine, 6(3), 351–355.
Kotamraju, S., Chitambar, C. R., Kalivendi, S. V., Joseph, J., & Kalyanaraman, B. (2002). Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. The Journal of Biological Chemistry, 277(19), 17179–17187.
Cohen, B., Dafni, H., Meir, G., Harmelin, A., & Neeman, M. (2005). Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia, 7(2), 109–117.
Naumova, A. V., Reinecke, H., Yarnykh, V., Deem, J., Yuan, C., & Murry, C. E. (2010). Ferritin overexpression for noninvasive magnetic resonance imaging-based tracking of stem cells transplanted into the heart. Molecular Imaging, 9(4), 201–210.
Campan, M., Lionetti, V., Aquaro, G. D., et al. (2011). Ferritin as a reporter gene for in vivo tracking of stem cells by 1.5-T cardiac MRI in a rat model of myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology, 300(6), H2238–H2250.
Gossuin, Y., Muller, R. N., & Gillis, P. (2009). Magnetic resonance imaging of cells overexpressing MagA, an endogenous contrast agent for live cell imaging. Molecular Imaging, 8(3), 129–139.
Zurkiya, O., Chan, A. W., & Hu, X. (2008). MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magnetic Resonance in Medicine, 59(6), 1225–1231.
Goldhawk, D. E., Lemaire, C., McCreary, C. R., et al. (2009). Magnetic resonance imaging of cells overexpressing MagA, an endogenous contrast agent for live cell imaging. Molecular Imaging, 8(3), 129–139.
McMahon, M. T., Gilad, A. A., DeLiso, M. A., Berman, S. M., Bulte, J. W., & van Zijl, P. C. (2008). New “multicolor” polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI. Magnetic Resonance in Medicine, 60(4), 803–812.
Alauddin, M. M., Shahinian, A., Gordon, E. M., & Conti, P. S. (2004). Direct comparison of radiolabeled probes FMAU, FHBG, and FHPG as PET imaging agents for HSV1-tk expression in a human breast cancer model. Molecular Imaging, 3(2), 76–84.
Koehne, G., Doubrovin, M., Doubrovina, E., et al. (2003). Serial in vivo imaging of the targeted migration of human HSV-TK-transduced antigen-specific lymphocytes. Nature Biotechnology, 21(4), 405–413.
Ponomarev, V., Doubrovin, M., Lyddane, C., et al. (2001). Imaging TCR-dependent NFAT-mediated T-cell activation with positron emission tomography in vivo. Neoplasia, 3(6), 480–488.
Pei, Z., Lan, X., Cheng, Z., et al. (2012). A multimodality reporter gene for monitoring transplanted stem cells. Nuclear Medicine and Biology, 39(6), 813–820.
Pomper, M. G., Hammond, H., Yu, X., et al. (2009). Serial imaging of human embryonic stem-cell engraftment and teratoma formation in live mouse models. Cell Research, 19(3), 370–379.
Mercier-Letondal, P., Deschamps, M., Sauce, D., et al. (2008). Early immune response against retrovirally transduced herpes simplex virus thymidine kinase-expressing gene-modified T cells coinfused with a T cell-depleted marrow graft: an altered immune response? Human Gene Therapy, 19(9), 937–950.
Serganova, I., Ponomarev, V., & Blasberg, R. (2007). Human reporter genes: potential use in clinical studies. Nuclear Medicine and Biology, 34(7), 791–807.
Campbell, D. O., Yaghoubi, S. S., Su, Y., et al. (2012). Structure-guided engineering of human thymidine kinase 2 as a positron emission tomography reporter gene for enhanced phosphorylation of non-natural thymidine analog reporter probe. The Journal of Biological Chemistry, 287(1), 446–454.
Ponomarev, V., Doubrovin, M., Shavrin, A., et al. (2007). A human-derived reporter gene for noninvasive imaging in humans: mitochondrial thymidine kinase type 2. Journal of Nuclear Medicine, 48(5), 819–826.
Yaghoubi, S. S., Jensen, M. C., Satyamurthy, N., et al. (2009). Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nature Clinical Practice Oncology, 6(1), 53–58.
McCracken, M. N., Gschweng, E. H., Nair-Gill, E., et al. (2013). Long-term in vivo monitoring of mouse and human hematopoietic stem cell engraftment with a human positron emission tomography reporter gene. Proceedings of the National Academy of Sciences of the United States of America, 110(5), 1857–1862.
Huang, M., Batra, R. K., Kogai, T., et al. (2001). Ectopic expression of the thyroperoxidase gene augments radioiodide uptake and retention mediated by the sodium iodide symporter in non-small cell lung cancer. Cancer Gene Therapy, 8(8), 612–618.
Auricchio, A., Acton, P. D., Hildinger, M., et al. (2003). In vivo quantitative noninvasive imaging of gene transfer by single-photon emission computerized tomography. Human Gene Therapy, 14(3), 255–261.
Kim, Y. H., Lee, D. S., Kang, J. H., et al. (2005). Reversing the silencing of reporter sodium/iodide symporter transgene for stem cell tracking. Journal of Nuclear Medicine, 46(2), 305–311.
Terrovitis, J., Kwok, K. F., Lautamaki, R., et al. (2008). Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. Journal of the American College of Cardiology, 52(20), 1652–1660.
Hwang do, W., Kang, J. H., Jeong, J. M., et al. (2008). Noninvasive in vivo monitoring of neuronal differentiation using reporter driven by a neuronal promoter. European Journal of Nuclear Medicine and Molecular Imaging, 35(1), 135–145.
Bar-Shir, A., Liu, G., Liang, Y., et al. (2013). Transforming thymidine into a magnetic resonance imaging probe for monitoring gene expression. Journal of the American Chemical Society, 135(4), 1617–1624.
de Vries, I. J., Lesterhuis, W. J., Barentsz, J. O., et al. (2005). Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nature Biotechnology, 23(11), 1407–1413.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srivastava, A.K., Bulte, J.W.M. Seeing Stem Cells at Work In Vivo. Stem Cell Rev and Rep 10, 127–144 (2014). https://doi.org/10.1007/s12015-013-9468-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-013-9468-x