Abstract
Purpose review
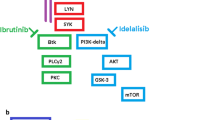
B cell signaling agents, including ibrutinib, idelalisib, and the BCL-2 inhibitor venetoclax have become an integral part of therapy for patients with non-Hodgkin’s lymphomas. The toxicity profiles of these medications is distinct from chemoimmunotherapy. Here, we will review the mechanism of action of these drugs, their efficacy, and toxicity management.
Recent findings
Ibrutinib use is associated with increased risk of atrial fibrillation and bleeding which can be managed using dose interruptions and modifications. Patients on idelalisib require close clinical and frequent laboratory monitoring, particularly of liver function tests to ensure there are no serious adverse events. Monitoring for infections is important in patients on both idelalisib and ibrutinib. Venetoclax requires close clinical and laboratory monitoring to prevent significant tumor lysis.
Summary
Targeted B cell receptor therapies each have unique side effect profiles which require careful clinical monitoring. As we continue to use these therapies, optimal management strategies will continue to be elucidated.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6(4):283–94. https://doi.org/10.1038/nri1808.
Arana E, Harwood NE, Batista FD. Regulation of integrin activation through the B-cell receptor. J Cell Sci. 2008;121(Pt 14):2279–86. https://doi.org/10.1242/jcs.017905.
Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34(12):592–601. https://doi.org/10.1016/j.it.2013.07.002.
Chantry D, Vojtek A, Kashishian A, Holtzman DA, Wood C, Gray PW, et al. p110δ, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem. 1997;272(31):19236–41. https://doi.org/10.1074/jbc.272.31.19236.
Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MJ, et al. p110δ, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci. 1997;94(9):4330–5.
Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–88. https://doi.org/10.1182/blood-2010-02-271171.
Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–12. https://doi.org/10.1182/blood-2011-05-352492.
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107(29):13075–80. https://doi.org/10.1073/pnas.1004594107.
Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–23. https://doi.org/10.1056/NEJMoa1400376.
• Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. https://doi.org/10.1056/NEJMoa1215637. This is the Phase 1b/2 study of ibrutinib in relapsed/refractory in patients with CLL which led to the accelerated approval of the drug. The overall response rate was 71% in patients receiving 420 mg or 840 mg daily.
Montillo M, Byrd JC, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, et al. Long-term efficacy and safety in the resonate study: ibrutinib in patients with previously treated chronic lymphocytic leukemia (CLL) with up to four years follow-up. Hematol Oncol. 2017;35:235–6. https://doi.org/10.1002/hon.2438_98.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37. https://doi.org/10.1056/NEJMoa1509388.
Tedeschi A, Owen CJ, Robak T, Barr PM, Bairey O, Hillmen P, et al. Prolonged improvement in patient-reported outcomes (PROs) and well-being in older patients with treatment-Naïve (TN) chronic lymphocytic leukemia treated with Ibrutinib (Ibr): 3-year follow-up of the RESONATE-2 study. Blood. 2017;130(Suppl 1):1746.
Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–11. https://doi.org/10.1016/S1470-2045(15)00465-9.
• Thompson PA, Levy V, Tam CS, Al Nawakil C, Goudot FX, Quinquenel A, et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175(3):462–6. https://doi.org/10.1111/bjh.14324. This is a retrospective cohort study which looked at 56 patients who developed atrial fibrillation while receiving treatment with ibrutinib. 91% of patients were treated with antiarrhythmic medications, 48% were started on anticoagulation, and ultimately 39% of patients discontinued ibrutinib.
Farooqui M, Valdez J, Soto S, Bray A, Tian X, Wiestner A. Atrial fibrillation in CLL/SLL patients on Ibrutinib. Blood. 2015;126(23):2933.
Mato A, Nabhan C, Kay NE, Weiss MA, Lamanna N, Kipps TJ, et al. Real-world clinical experience in the connect(R) chronic lymphocytic leukaemia registry: a prospective cohort study of 1494 patients across 199 US centres. Br J Haematol. 2016;175(5):892–903. https://doi.org/10.1111/bjh.14332.
McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–30. https://doi.org/10.1182/blood-2014-10-604272.
Byrd JC, Hillmen P, James DF. Response: additional data needed for a better understanding of the potential relationship between atrial fibrillation and ibrutinib. Blood. 2015;125(10):1673. https://doi.org/10.1182/blood-2015-01-621466.
• Leong DP, Caron F, Hillis C, Duan A, Healey JS, Fraser G, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138–40. https://doi.org/10.1182/blood-2016-05-712828. This is a systematic review and meta-analysis of 20 manuscripts (4 RCTs ,10 Phase II studies, 1 prospective cohort study, 5 retrospective cohort studies) which looked at the rates of atrial fibrillation in patients treated with ibruitnib (pooled relative risk 3.9).
Mato AR, Clasen S, Pickens P, Gashonia L, Rhodes J, Svoboda J, et al. Left atrial abnormality (LAA) as a predictor of ibrutinib-associated atrial fibrillation in patients with chronic lymphocytic leukemia. Cancer Biol Ther. 2017;19:1–2. https://doi.org/10.1080/15384047.2017.1394554.
Vrontikis A, Carey J, Gilreath JA, Halwani A, Stephens DM, Sweetenham JW. Proposed algorithm for managing ibrutinib-related atrial fibrillation. Oncology (Williston Park). 2016;30(11):970–4. 80–1, C3
Janssen. Ibrutinib Prescribing Information 2017. https://www.imbruvica.com/docs/librariesprovider7/default-document-library/prescribing_information.pdf.
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. https://doi.org/10.1016/j.jacc.2014.03.022.
de Zwart L, Snoeys J, De Jong J, Sukbuntherng J, Mannaert E, Monshouwer M. Ibrutinib dosing strategies based on interaction potential of CYP3A4 perpetrators using physiologically based pharmacokinetic modeling. Clin Pharmacol Ther. 2016;100(5):548–57. https://doi.org/10.1002/cpt.419.
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. https://doi.org/10.1378/chest.09-1584.
Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739–45. https://doi.org/10.1182/blood-2015-03-635326.
Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124(26):3991–5. https://doi.org/10.1182/blood-2014-06-583294.
Oda A, Ikeda Y, Ochs HD, Druker BJ, Ozaki K, Handa M, et al. Rapid tyrosine phosphorylation and activation of Bruton’s tyrosine/Tec kinases in platelets induced by collagen binding or CD32 cross-linking. Blood. 2000;95(5):1663–70.
Lipsky AH, Farooqui MZ, Tian X, Martyr S, Cullinane AM, Nghiem K, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571–8. https://doi.org/10.3324/haematol.2015.126672.
Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29(4):783–7. https://doi.org/10.1038/leu.2014.247.
• Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Advances. 2017;1(12):772–8. https://doi.org/10.1182/bloodadvances.2016001883. This is a systematic review and meta-analysis of 22 studies (obsevation, randomized control trials, prostpective cohort, and retrospective cohorts) which looked at risk of overall bleeding and major bleeding with ibrutinib as compared to alternative treatment strategies. They found there was an increased risk of overall bleeding (RR 2.72) but not of major bleeding (RR 1.66) with treatment with ibrutinib.
Jones JA, Hillmen P, Coutre S, Tam C, Furman RR, Barr PM, et al. Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib. Br J Haematol. 2017;178(2):286–91. https://doi.org/10.1111/bjh.14660.
Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med. 2015;372(15):1430–40. https://doi.org/10.1056/NEJMoa1501548.
Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–506. https://doi.org/10.1182/blood-2014-10-606038.
• Mato AR, Hill BT, Lamanna N, Barr PM, Ujjani CS, Brander DM, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multi-center study of 683 patients. Ann Oncol. 2017; https://doi.org/10.1093/annonc/mdw. This is the largest cohort study which looked at outcomes, adverse events, reasons for drug discontinuation, and subsequent lines of therapy in patients treated with kinase inhibitors.
Forum CGUC. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–72. https://doi.org/10.3324/haematol.2016.147900.
Mato AR, Islam P, Daniel C, Strelec L, Kaye AH, Brooks S, et al. Ibrutinib-induced pneumonitis in patients with chronic lymphocytic leukemia. Blood. 2016;127(8):1064–7. https://doi.org/10.1182/blood-2015-12-686873.
• Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. https://doi.org/10.1056/NEJMoa1315226. This is a Phase 3 study of idelalisib/rituximab vs. rituximab/placebo in patients with relapsed/refractory CLL. Median PFS was not reached in the idelalisib arm vs 5.5 months in the placebo arm.
Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with Ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis. 2018;66(1):140–8. https://doi.org/10.1093/cid/cix687.
Rogers KA, Luay M, Zhao Q, Wiczer T, Levine L, Zeinab EB, et al. Incidence and type of opportunistic infections during Ibrutinib treatment at a single academic center. Blood. 2017;130(Suppl 1):830.
Tillman BF, Pauff JM, Satyanarayana G, Talbott M, Warner JL. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur J Haematol. 2018;2017 https://doi.org/10.1111/ejh.13020.
Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–32. https://doi.org/10.1056/NEJMoa1509981.
Harrington BK, Gulrajani M, Covey T, Kaptein A, Van Lith B, Izumi R, et al. ACP-196 is a second generation inhibitor of Bruton tyrosine kinase (BTK) with enhanced target specificity. Blood. 2015;126(23):2908.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas R-O, Smith SD, et al. Efficacy and safety of Acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma in the phase 2 ACE-LY-004 study. Blood. 2017;130(Suppl 1):155.
Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22(7):267–72.
Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–18. https://doi.org/10.1056/NEJMoa1314583.
Inc GS. Zydelig full prescribing information 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206545lbl.pdf. Accessed 4 Oct 2017.
• Coutre SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10):2779–86. https://doi.org/10.3109/10428194.2015.1022770. This is review of an expert panel of the management of idealisib associated toxicities in particular management of diarrhea and colitis.
O'Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–94. https://doi.org/10.1182/blood-2015-03-630947.
Thompson PA, Stingo F, Keating MJ, Ferrajoli A, Burger JA, Wierda WG, et al. Outcomes of patients with chronic lymphocytic leukemia treated with first-line idelalisib plus rituximab after cessation of treatment for toxicity. Cancer. 2016;122(16):2505–11. https://doi.org/10.1002/cncr.30069.
Weidner AS, Panarelli NC, Geyer JT, Bhavsar EB, Furman RR, Leonard JP, et al. Idelalisib-associated colitis: histologic findings in 14 patients. Am J Surg Pathol. 2015;39(12):1661–7. https://doi.org/10.1097/PAS.0000000000000522.
Louie CY, DiMaio MA, Matsukuma KE, Coutre SE, Berry GJ, Longacre TA. Idelalisib-associated enterocolitis: Clinicopathologic features and distinction from other Enterocolitides. Am J Surg Pathol. 2015;39(12):1653–60. https://doi.org/10.1097/PAS.0000000000000525.
Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128(2):195–203. https://doi.org/10.1182/blood-2016-03-707133.
Jin F, Robeson M, Zhou H, Hisoire G, Ramanathan S. The pharmacokinetics and safety of idelalisib in subjects with severe renal impairment. Cancer Chemother Pharmacol. 2015;76(6):1133–41. https://doi.org/10.1007/s00280-015-2898-1.
Zelenetz AD, Barrientos JC, Brown JR, Coiffier B, Delgado J, Egyed M, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18(3):297–311. https://doi.org/10.1016/S1470-2045(16)30671-4.
Goldring L, Kumar B, Gan TE, Low MSY. Idelalisib induced CMV gastrointestinal disease: the need for vigilance with novel therapies. Pathology. 2017;49(5):555–7. https://doi.org/10.1016/j.pathol.2017.03.009.
Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128(3):331–6. https://doi.org/10.1182/blood-2016-02-702761.
Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10(3):456–9.
Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117(1):112–21. https://doi.org/10.1172/JCI28281.
Abbvie. Venclexta Full Prescribing Information 2017. http://www.rxabbvie.com/pdf/venclexta.pdf.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8. https://doi.org/10.1038/nm.3048.
Perez-Galan P, Roue G, Lopez-Guerra M, Nguyen M, Villamor N, Montserrat E, et al. BCL-2 phosphorylation modulates sensitivity to the BH3 mimetic GX15-070 (Obatoclax) and reduces its synergistic interaction with bortezomib in chronic lymphocytic leukemia cells. Leukemia. 2008;22(9):1712–20. https://doi.org/10.1038/leu.2008.175.
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–8. https://doi.org/10.1158/0008-5472.CAN-07-5836.
Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11(12):1149–59. https://doi.org/10.1016/S1470-2045(10)70261-8.
• Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–22. https://doi.org/10.1056/NEJMoa1513257. This is the Phase 1 study of venetoclax in patients with relapsed/refractory CLL.They demonstrated that with the adjusted dose-escalation schedule, venetoclax could be safely administered.
• Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–78. https://doi.org/10.1016/S1470-2045(16)30019-5. This is the Phase 2 trial of venetoclax in patients with relpased/refactory del17p CLL. They demonstrated an ORR 85%, which led to the approval of venetoclax for patients with relapsed/refractory del17p CLL.
Seymour JF. Effective mitigation of tumor lysis syndrome with gradual venetoclax dose ramp, prophylaxis, and monitoring in patients with chronic lymphocytic leukemia. Ann Hematol. 2016;95(8):1361–2. https://doi.org/10.1007/s00277-016-2695-x.
Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844–54. https://doi.org/10.1056/NEJMra0904569.
Roberts AW, Stilgenbauer S, Seymour JF, Huang DCS. Venetoclax in patients with previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(16):4527–33. https://doi.org/10.1158/1078-0432.CCR-16-0955.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Joanna Rhodes declares that she has no conflict of interest.
Anthony Mato has received research funding from Portola, AbbVie, Acerta, DTRM, TG Therapeutics, Pharmacyclics, and Regeneron; has received compensation from AbbVie, AstraZeneca, Janssen, and Kite for service as a consultant; and has served on advisory boards for Gilead, TG Therapeutics, and Celgene.
Jeff P. Sharman has received research funding from AbbVie, Gilead, Acerta, TG Therapeutics, and Pharmacyclics; has received compensation from AbbVie, Gilead, Acerta, TG Therapeutics, and Pharmacyclics for service as a consultant; and has served on an advisory board for Genentech.
Human and Animal Rights and Informed Consent
This article contains studies with human or animal subjects performed by the authors.
Additional information
This article is part of the Topical Collection on Lymphomas
Rights and permissions
About this article
Cite this article
Rhodes, J., Mato, A. & Sharman, J.P. Monitoring and Management of Toxicities of Novel B Cell Signaling Agents. Curr Oncol Rep 20, 49 (2018). https://doi.org/10.1007/s11912-018-0694-x
Published:
DOI: https://doi.org/10.1007/s11912-018-0694-x