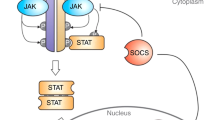
Abstract
Over the last decade, unparalleled advances have been made within the field of ‘Philadelphia chromosome’-negative myeloproliferative neoplasms (MPN) regarding both disease pathogenesis and therapeutic targeting. The discovery of deregulated JAK-STAT signalling in MPN led to the rapid development of JAK inhibitor agents, targeting both mutated and wild-type JAK, which have significantly altered the therapeutic paradigm for patients with MPN. Although the largest population treated with these agents incorporates those with myelofibrosis, increasing data supports potential usage in other MPNs such as essential thromocythaemia and polycythaemia vera. Many MPNs are associated with a hyperinflammatory state and deregulation of immune homeostasis. Over the last few years, research has focused on attempting to decipher the complex and context-dependent changes that contribute to this immune deregulation. Moreover, very recent studies have demonstrated significant JAK inhibitor-mediated effects within the T cell, natural killer cell and dendritic cell compartments following exposure to JAK inhibitors. In parallel, case reports of infections occurring following exposure to ruxolitinib, many of which are atypical, have focused research efforts on delineating JAK inhibitor-associated immunological consequences. Within this review article, we will describe what is currently known about MPN-associated immune deregulation and JAK inhibitor-mediated immunomodulation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythaemia vera. Nature. 2005;434(7037):1144–8.
Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):779–90.
Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97.
Baxter EJ, Scott LM, Campbell PJ, et al. Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. Erratum in: Lancet 2005; 366(9480)122.
Quintas-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109–17.
Tefferi A, Gilliland DG. Oncogenes in myeloproliferative disorders. Cell Cycle. 2007;6:550–66.
Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128–38.
Larsen TS, Christensen JH, Hasselbalch HC, Pallisgaard N. The JAK2 V617F mutation involves B- and T-lymphocyte lineages in a subgroup of patients with Philadelphia-chromosome negative chronic myeloproliferative disorders. Br J Haematol. 2007;136(5):745–51.
Delhommeau F, Dupont S, Tonetti C, et al. Evidence that the JAK2 G1849T (V617F) mutation occurs in a lympho-myeloid progenitor in polycythemia vera and idiopathic myelofibrosis. Blood. 2006;109:71–7.
United States full prescribing information. Jakafi (ruxolitinib). Revised 11/2011. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf.
EMA Summary of Product Characteristics. Jakavi. Novartis Pharmaceuticals UK Ltd. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/002464/WC500133223.pdf. Accessed 3/12/2014
Harrison C, Kiladjian J-J, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. COMFORT-II trial.
Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. COMFORT-I trial.
Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–35.
Eghtedar A, Verstovsek S, Estrov Z, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119:4614–8.
Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167(3):668–77.
Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–9.
Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–86.
Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with ruxolitinib, a novel Janus kinase 1,2 inhibitor. Chest. 2013;143:1478–9.
Goldberg RA, Reichel E, Oshry LJ. Bilateral toxoplasmosis retinitis associated with ruxolitinib. N Engl J Med. 2013;369:681–3.
Caocci G, Murgia F, Podda L, Solinas A, Atzeni S, La Nasa G. Reactivation of hepatitis B virus infection following ruxolitinib treatment in a patient with myelofibrosis. Leukemia. 2014;28:225–7.
Wathes R, Moule S, Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N Engl J Med. 2013;369:197–8.
Yamaoka K, Saharinen P, Pesu M, Holt VE, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks). Genome Biol. 2004;5(12):253.
Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–87.
Müller M, Briscoe J, Laxton C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993;366(6451):129–35.
Schindler C, Levy D, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059–63.
Krishnan K, Pine R, Krolewski JJ. Kinase-deficient forms of Jak1 and Tyk2 inhibit interferon signaling in a dominant manner. Eur J Biochem. 1997;247:298–305.
O’Sullivan LA, Liongue C, Lewis RS, Stephenson SE, Ward AC. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Mol Immunol. 2007;44(10):2497–506.
Waters MJ, Brooks AJ. JAK2 activation by growth hormone and other cytokines. Biochem J. 2015;466(1):1–11.
Macchi P, Villa A, Giliani S, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature. 1995;377:65.
Ishizaki M, Akimoto T, Muromoto R, et al. Involvement of tyrosine kinase-2 in both theIL-12/Th1 and IL-23/Th17 axes in vivo. J Immunol. 2011;187(1):181–9.
Tokumasa N, Suto A, Kagami S, et al. Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood. 2007;110(2):553–60.
Prchal-Murphy M, Witalisz-Siepracka A, Bednarik K, et al. In vivo tumor surveillance by NK cells requires TYK2 but not TYK2 kinase activity. Oncoimmunology. 2015.
Agnello D, Lankford CS, Bream J, et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23(3):147–61.
Yao Z, Kanno Y, Kerenyi M, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109(10):4368–75.
Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–63.
Barosi G. An immune dysregulation in MPN. Curr Hematol Malig Rep. 2014;9:331–9.
Kristinsson SY, Landgren O, Samuelsson J, Björkholm M, Goldin LR. Autoimmunity and the risk of myeloproliferative neoplasms. Haematologica. 2010;95(7):1216–20.
Barcellini W, Iurlo A, Radice T, et al. Increased prevalence of autoimmune phenomena in myelofibrosis: relationship with clinical and morphological characteristics, and with immunoregulatory cytokine patterns. Leuk Res. 2013;37(11):1509–15.
Skov V, Larsen TS, Thomassen M, et al. Molecular profiling of peripheral blood cells from patients with polycythemia vera and related neoplasms: identification of deregulated genes of significance for inflammation and immune surveillance. Leuk Res. 2012;36(11):1387–92.
Skov V, Riley CH, Thomassen M, et al. Whole blood transcriptional profiling reveals significant down-regulation of human leukocyte antigen class I and II genes in essential thrombocythemia, polycythemia vera and myelofibrosis. Leuk Lymphoma. 2013;54(10):2269–73.
Tefferi A, Vaidya R, Caramazza D, et al. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–63.
Vannucchi AM, Bianchi L, Paoletti F, et al. A pathologic pathway linking thrombopoietin, GATA-1 and TGF-β1 in the development of myelofibrosis. Blood. 2005;105(9):3493–501.
Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61.
Kundra A, Baptiste S, Chen C, Sindhu H and Wang JC. Programmed cell death receptor (PD-1), PD-1 ligand (PD-L1) expression and myeloid derived suppressor cells (MDSC) in myeloid neoplasms implicate the mechanism of IMiD treatment of myelofibrosis. Blood (supplement) 2013; abstract 2837.
Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–53.
Wilkins BS, Radia D, Woodley C, et al. Resolution of bone marrow fibrosis in a patient receiving JAK1/JAK2 inhibitor treatment with ruxolitinib. Haematologica. 2013;98(12):1872–6.
Lee SC, Feenstra J, Georghiou PR. Pneumocystis jiroveci pneumonitis complicating ruxolitinib therapy. BMJ Case Rep. 2014. 2014.
Landman GW, Arend SM, van Dissel JT. Ruxolitinib can mask symptoms and signs of necrotizing fasciitis. J Infect. 2013;66(3):296–7.
Kim Y-K, Lee SR, Park Y, et al. Efficacy of ruxolitinib in Korean myelofibrosis patients and cases complicated TB lymphadenitis during the treatment. Blood. 2013;122:1596.
Mesa R, Egyed M, Szoke A et al. Results of the PERSIST-1 phase III study of pacritinib (PAC) versus best available therapy (BAT) in primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF), or post-essential thrombocythemia-myelofibrosis (PET-MF). J Clin Oncol 33, 2015 (suppl; abstr LBA7006)
Singer J, Al-Fayoumi S, Ma H et al. Comprehensive kinase profile of pacritinib, a non-myelosuppressive JAK2 kinase inhibitor in phase 3 development in primary and post ET/PV myelofibrosis. [abstract] 2013; Blood: 122 (21) 1874.
Pardanani A, Harrison C, Cortes J et al. Results of a randomized, double-blind, placebo-controlled phase III study (JAKARTA) of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis (MF). [abstract] 2013; Blood: 122 (21) 393.
Zhang Q, Zhang Y, Diamond S, et al. The Janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: a putative mechanism for the onset of Wernicke’s encephalopathy. Drug Metab Dispos. 2014;42(10):1656–62.
Pardanani A, Gotlib J, Gupta V et al. Update on the long-term efficacy and safety of momelotinib, a JAK1 and JAK2 Iinhibitor, for the treatment of myelofibrosis. [abstract] 2013; Blood: 122 (21) 108.
Verstovsek S, Mesa R, Salama M et al. Phase I study of LY2784544, a JAK2 selective inhibitor, in patients with myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET). [abstract] 2013; Blood: 122 (21).
Meyer DM, Jesson MI, Li X, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond). 2010;7:41.
Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60(7):1895–905.
Cohen S, Radominski SC, Gomez-Reino JJ, Wang L, Krishnaswami S, Wood SP, et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(11):2924–37.
Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, long term extension studies. J Rheumatol. 2014;41(5):837–52.
Vincenti F, Tedesco Silva H, Busque S, et al. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant : Off J Am Soc Transplant Am Soc Transplant Surg. 2012;12:2446–56.
Massa M, Rosti V, Campanelli R, Fois G, Barosi G. Rapid and long-lasting decrease of T-regulatory cells in patients with myelofibrosis treated with ruxolitinib. Leukemia. 2014;28(2):449–51.
Keohane C, Kordasti SY, Siedl T et al. JAK inhibition reduces CD25 high CD27+ FOXp3+ T regulatory cells and causes a silencing of T effector cells in patients with myeloproliferative neoplasms whilst promoting a TH17 phenotype. Abstract 4092 Blood 2013
Sharma MD, Hou DY, Baban B, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33(6):942–54.
Parampalli Yajnanarayana S, Stübig T, Cornez I, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol. 2015 [Epub ahead of print]
Perner F, Saalfeld F, Schnoeder T et al. Specificity of JAK-kinase inhibition determines impact on T-cell function. [abstract] 2013; Blood: 122 (21) 1410.
Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9.
Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. Review.
Radaev S, Sun PD. Structure and function of natural killer cell surface receptors. Annu Rev Biophys Biomol Struct. 2003;32:93–114. Epub 2002. Review. 32, 93-114.
De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A. 2011;108(2):728–32.
Fauriat C, Long EO, Ljunggren H-G, Bryceson YT. Regulation of human NK cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–76.
Gersuk GM, Carmel R, Pattamakom S, Challita PM, Rabinowitz AP, Pattengale PK. Quantitative and functional studies of impaired natural killer (NK) cells in patients with myelofibrosis, essential thrombocythemia, and polycythemia vera. I. A potential role for platelet-derived growth factor in defective NK cytotoxicity. Nat Immun. 1993;12(3):136–51.
Riley CH, Hansen M, Brimnes MK, et al. Expansion of circulating CD56bright natural killer cells in patients with JAK2-positive chronic myeloproliferative neoplasms during treatment with interferon-α. Eur J Haematol. 2015;94(3):227–34.
Schönberg K, Rudolph J, Vonnahme M et al. JAK inhibition impairs NK cell function in myeloproliferative neoplasms. Cancer Res. 2015.
Merad M, Manz M. Dendritic cell homeostasis. Blood. 2009;113(15):3418–27.
Watowich S, Liu YJ. Mechanisms regulating dendritic cell specification and development. J Exp Med. 2003;198(2):305–13.
Li HS, Watowich SS. Diversification of dendritic cell subsets: emerging roles for STAT proteins. JAKSTAT. 2013;2(4):e25112.
Zhong J, Yang P, Muta K, et al. Loss of Jak2 selectively suppresses DC-mediated innate immune response and protects mice from lethal dose of LPS-induced septic shock. PLoS ONE. 2010;5(3):e9593.
Heine A, Held SA, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192–202.
Kubo S, Yamaoka K, Kondo M, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis. 2014;73(12):2192–8.
Spoer S, Mathew N, Bscheider M et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. 2014; Blood: 123 (24).
Teshima T. JAK inhibitors: a home run for GVHD patients? Blood. 2014;123(24):3691–3.
Spoerl S, Maas-Bauer K, Verbeek M et al. Response to JAK 1/2 inhibition in patients with corticosteroid-refractory acute graft-versus-host disease. ASH 2014 meeting abstract
Carniti C, Gimondi S, Vendramin A, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015.
Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–55.
Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169(1):137–45.
Hsu L, Armstrong A. JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014:283617.
Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67(4):658–64.
Compliance with Ethics Guidelines
Conflict of Interest
Donal P. McLornan reports honoraria, speaker’s fees paid and travel expenses covered from Novartis.
Alesia A. Khan declares no potential conflicts of interest.
Claire N. Harrison is a section editor for Current Hematologic Malignancy Reports.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors. Where work by the authors concerning human subjects is quoted, this was with full ethical approval and informed consent
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Myeloproliferative Disorders
Rights and permissions
About this article
Cite this article
McLornan, D.P., Khan, A.A. & Harrison, C.N. Immunological Consequences of JAK Inhibition: Friend or Foe?. Curr Hematol Malig Rep 10, 370–379 (2015). https://doi.org/10.1007/s11899-015-0284-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-015-0284-z