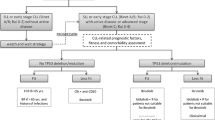
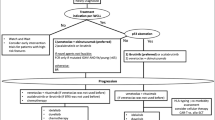
Opinion statement
At the end of the 1990s, with the advent of imatinib for chronic myeloid leukemia and rituximab for B cell lymphoproliferative diseases with CD20 expression, there was a great conceptual evolution in the treatment of onco-hematological diseases. Researchers from around the world and the pharmaceutical industry began to focus their efforts on the so-called target therapy used alone or associated with classic chemotherapeutic drugs. In chronic lymphocytic leukemia, the development of second-generation anti-CD20 antibodies, biosimilars, PI3K (phosphatidylinositol 3-kinases) inhibitors, BTK (Bruton’s tyrosine kinase) inhibitors, and anti-bcl 2 drugs represented mainly by venetoclax brought new, broader, and more effective opportunities in the treatment of this disease. This breakthrough occurred mainly regarding patients with alteration in 17p or mutation of the p53 gene for whom selecting the new drugs that act on B cell signaling (BTK and PI3K inhibitors) in the first line is mandatory. In fit patients with immunoglobulin heavy chain mutation, it is still acceptable to use the chemotherapy regimen with fludarabine, cyclophosphamide, and rituximab (FCR) and, in those who do not fit or are not IgVH-mutated, bendamustine-rituximab regimen. However, the first-line use of ibrutinib or venetoclax associated with immunotherapy within the concepts of infinite (ibrutinib) or finite (venetoclax) treatment has been increasingly used. In the second line, venetoclax, ibrutinib, and idelalisib have become the preferred treatments. I believe that a process of instruction and decision shared with patients considering the risks–benefits–cost and access to treatments should guide the choices within these concepts. Another fundamental aspect to discuss is the objective of the treatment for chronic lymphocytic leukemia (CLL) for a specific patient: the increase progression-free survival and overall survival and/or the achievement of minimal residual disease. CLL is the most common leukemia in adults with a median age at diagnosis of 72 years. The clinical course is heterogeneous, and outcomes are influenced by individual clinical presentation and disease biology. Molecular and genomic factors, including fluorescence in situ hybridization (FISH) testing, karyotype, and immunoglobulin heavy chain variable region gene (IGHV) mutational status, are important to treatment decisions and to predict the clinical course. However, despite disease biology, the presence of active disease is the most important criteria to initiate treatment. In the past decade, target therapies that inhibit B cell receptor signaling pathways and, more recently, BCL2 antagonists have emerged as a new treatment paradigm: chemo-free with fixed duration therapy. Bruton’s tyrosine kinase inhibitors (BTK) are a class of oral medications approved for frontline and relapsed disease, effective for achieving lasting response and disease control with a good safety profile. BTK inhibitors are an attractive option for high-risk patients who are not candidates for an intensive regimen. However, it is a continuous therapy, and drug resistance or severe adverse events could lead to treatment suspension. BCL2 antagonists are an attractive alternative to BTK inhibitors. Anti-apoptotic BCL2 is associated with tumor genesis and chemotherapy resistance. The BCl2, an anti-apoptotic protein located in the mitochondrial membrane, is a major contributor to the pathogenesis of lymphoid malignancies and is overexpressed in CLL cells promoting clonal cell survival. Venetoclax is a potent and selective member of the BH3 mimetic drugs and a physiologic antagonist of BCL2. Venetoclax has demonstrated quick and durable responses in naïve and relapsed or refractory CLL (r/r CLL) patients, including high-risk patients. Furthermore, it has shown deeper responses, achieving a higher incidence of negative minimal residual disease (MRD) with a fixed duration therapy. In the past decade, there was a remarkable progress in CLL treatment. However, neither of the new target therapies is considered curative or free of toxicity. This article will focus on the treatment approach of CLL patients with BCl2 antagonists. Treatment strategy (combined versus monotherapy; continuous versus limited duration therapy), toxicity profile, and future directions will be exposed in this review.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Burger JA. Treatment of chronic lymphocytic leukemia. N Engl J Med. 2020;383(5):460–73. https://doi.org/10.1056/nejmra1908213.
International CLL-IPI Working Group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;17:779–90. https://doi.org/10.1016/s1470-2045(16)30029-8. Describes the importance of citogenetic markers in CLL treatment decision.
Roberts AW, Ma S, Kipps TJ, Coutre SE, Davids MS, Eichhorst B, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood. 2019;134(2):111–22. https://doi.org/10.1182/blood.2018882555.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–28. https://doi.org/10.1056/nejmoa1812836.
Crombie J, Davids MS. Venetoclax for the treatment of patients with chronic lymphocytic leukemia. Future Oncol. 2017;13(14):1223–32. https://doi.org/10.2217/fon-2017-0031.
Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline, et al. Venetoclax–rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–20. https://doi.org/10.1056/NEJMoa1713976. Phase 3 trial of anti CD20 and venetoclax demonstrated a better disease control and durable responses comparing to chemo immunotherapy in pre treated CLL patients.
Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–36. https://doi.org/10.1056/NEJMoa1815281.
Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–56. https://doi.org/10.1038/nrc883.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–81. https://doi.org/10.1038/nature03579.
Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. Novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26(16):2374–80. https://doi.org/10.1038/sj.onc.1210028.
Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–88. https://doi.org/10.1016/j.ccr.2006.10.006.
Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–96. https://doi.org/10.1200/jco.2011.34.7898.
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–22. https://doi.org/10.1056/nejmoa1513257. First-in-human, phase I dose escalation study to establish venetoclax as a well-tolerated, efficacious treatment for patients with relapsed/refractory CLL.
Perini GF, Ribeiro GN, Pinto Neto JV, Campos LT, Hamerschlak N. BCL-2 as therapeutic target for hematological malignancies. J Hematol Oncol. 2018;11(1):65. https://doi.org/10.1186/s13045-018-0608-2.
Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826–33. https://doi.org/10.1200/JCO.2016.70.4320.
Agarwal SK, Salem AH, Danilov AV, Hu B, Puvvada S, Gutierrez M, et al. Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL-2 inhibitor, in patients with non-Hodgkin lymphoma. Br J Clin Pharmacol. 2017;83(4):846–54. https://doi.org/10.1111/bcp.13175.
Agarwal SK, Hu B, Chien D, Wong SL, Salem AH. Evaluation of rifampin’s transporter inhibitory and CYP3A inductive effects on the pharmacokinetics of venetoclax, a BCL-2 inhibitor: results of a single- and multiple-dose study. J Clin Pharmacol. 2016;56(11):1335–43. https://doi.org/10.1002/jcph.730.
Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, Phase 2 study. Lancet Oncol. 2016;17(6):768–78. https://doi.org/10.1016/s1470-2045(17)30012-8. This phase II study showed that venetoclax is highly efficacious in patients with high-risk CLL with del(17p).
Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18(2):230–40. First trial with venetoclax plus rituximab; results showed a deep response with durable disease control.
Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D’Rozario J, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO phase III study. J Clin Oncol. 2020:JCO.20.00948. https://doi.org/10.1200/jco.20.00948.
Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink A-M, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. The Lancet Oncology. 2020;21(9):1188–200. https://doi.org/10.1016/s1470-2045(20)30443-5.
Jones GL, Will A, Jackson GH, Webb NJ. Rule S; British Committee for Standards in Haematology. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169(5):661–71. https://doi.org/10.1111/bjh.13403.
Davids MS, Hallek M, Wierda W, Roberts AW, Stilgenbauer S, Jones JA, et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res. 2018;24(18):4371–9. https://doi.org/10.1158/1078-0432.CCR-17-3761.
Stilgenbauer S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36(19):1973–80. https://doi.org/10.1200/jco.2017.76.6840.
Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first- line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–98. https://doi.org/10.1038/s41375-019-0602-x.
Teh BW, Tam CS, Handunnetti S, Worth LJ, Slavin MA. Infections in patients with chronic lymphocytic leukaemia: mitigating risk in the era of targeted therapies. Blood Rev. 2018;32(6):499–507. https://doi.org/10.1016/j.blre.2018.04.007.
Moreno C. Standard treatment approaches for relapsed/refractory chronic lymphocytic leukemia after frontline chemoimmunotherapy. Hematology Am Soc Hematol Educ Program. 2020;2020(1):33–40. https://doi.org/10.1182/hematology.2020000086.
Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14. https://doi.org/10.1016/j.jval.2013.08.2291.
Davids MS, Chatterjee A, Ravelo A, Shapouri S, Manzoor BS, Sail K, et al. Cost-effectiveness of a 12-month fixed duration of venetoclax in combination with obinutuzumab in first-line chronic lymphocytic leukemia in the United States. Blood. 2019;134(suppl 1):4741. https://doi.org/10.1182/blood-2019-123706.
Cho SK, Manzoor BS, Sail KR, Parisé H, Ravelo A, Shapouri S, et al. Budget impact of 12-month fixed treatment duration venetoclax in combination with obinutuzumab in previously untreated chronic lymphocytic leukemia patients in the United States. PharmacoEconomics. 2020;38:941–51. https://doi.org/10.1007/s40273-020-00919-1.
Hillmen P, Rawstron AC, Brock K, Muñoz-Vicente S, Yates FJ, Bishop R, et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study. J Clin Oncol. 2019;37(30):2722–9. https://doi.org/10.1200/JCO.19.00894.
Tam CS, Siddiqi T, Allan JN, et al. Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): results from the MRD cohort of the phase 2 CAPTIVATE study. Blood. 2019;134, 35(Supplement_1). https://doi.org/10.1182/blood-2019-121424.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflicts of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Perini, G.F., Feres, C.C.P., Teixeira, L.L.C. et al. BCL-2 Inhibition as Treatment for Chronic Lymphocytic Leukemia. Curr. Treat. Options in Oncol. 22, 66 (2021). https://doi.org/10.1007/s11864-021-00862-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00862-z