Summary
Background
A prospective Phase II study of irinotecan (CPT-11) in adult patients with recurrent surgery and radiotherapy-refractory WHO Grade I meningioma.
Methods
Sixteen patients (5 men; 11 women) ages 48–70 years (median 62.5), with recurrent meningioma were treated. All patients had previously been treated with surgery (complete in 4; partial in 9; biopsy in 3) and involved-field radiotherapy (median dose 54 Gy; 12 following first surgery and 4 following second surgery). Additionally, eight patients underwent re-operation (complete in 2; partial in 6) and eight patients were treated with salvage stereotactic radiosurgery. No patient was treated with prior chemotherapy. CPT-11 was administered intravenously every 3 weeks (350 mg/m2/day in patients on non-enzyme inducing anticonvulsants [NEIAED]; 600 mg/m2/day in patients on enzyme-inducing anticonvulsants [EIAED]) for 9 weeks (operationally defined as a single cycle). Neurological and neuroradiographic evaluation were performed every 10 weeks.
Results
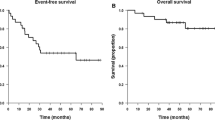
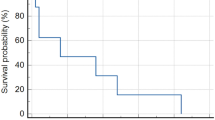
All patients were evaluable. A median of two cycles of CPT-11 (range 1–4) was administered. CPT-11 related-toxicity (≥grade 3) included diarrhea (6 occurrences, 19% all cycles administered), granulocytopenia (6,␣19%), leukopenia (5, 16%), thrombocytopenia (3, 10%) and anemia (3, 10%). Four patients required transfusion (3 RBC and 1 platelet). One patient developed neutropenic fever without bacteriologic confirmation. No treatment-related deaths occurred. No patient demonstrated a neuroradiographic complete or partial response (PR), 13 patients (81%) demonstrated stable disease but disease progressed after 2 cycles of CPT-11, and 3 patients (19%) had progressive disease (PD) following a single cycle of CPT-11. Time to tumor progression ranged from 2.5 to 5.0 months (median 5.0 months). Survival ranged from 4 to months (median 7.5 months).
Conclusions
The primary objective was to estimate the 6-month progression-free survival (PFS) after study entry. As no patient demonstrated PFS at 6-months, the study was stopped prematurely as specified by study design. Using CPT-11 in this moderately toxic dose schedule failed to demonstrate efficacy in this cohort of adult patients with recurrent surgery and radiotherapy-refractory meningioma.
Similar content being viewed by others
References
Bondy M, Ligon BL, Epidemiology and etiology of intracranial meningiomas. A review J Neuro-Oncol 29:197–205,1996
Wilson CB, Meningiomas: genetics, malignancy, and the role of radiation in induction and treatment J Neurosurg 81:666–675,1994
Braunstein JB, Vick NA, Meningiomas: the decision not to operate Neurology 48: 1459–1462, 1997
Firsching RP, Fischer A, Peters R, Thun F, Klug N, Growth rate of incidental meningiomas J Neurosurg 73:545–547, 1990
Go RS, Taylor BV, Kimmel DW, The natural history of asymptomatic meningiomas in Olmsted County, Minnesota Neurology 51:1718–1720, 1998
Goldsmith BJ, Wara WM, Wilson CB, Larson DA, Postoperative irradiation for subtotally resected meningiomas J Neurosurg 80:195–201, 1994
Mirimanoff RO, Dosoretz DE, Linggood RM, et al, Meningioma: analysis of recurrence and progression following neurosurgical resection J Neurosurg 62:18–24, 1985
Jaaskelainen J, Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis Surg Neurol 26: 261–469, 1986
Kondziolka D, Lunsford D, Coffey RJ, Flickinger JC, Stereotactic radiosurgery of meningiomas J Neurosurg 74:552–559, 1991
Lunsford DL, Contemporary management of meningiomas: radiation therapy as an adjuvant and radiosurgery as an alternative to surgical removal? J Neurosurg 80:187–190, 1994
Morita A, Coffey RJ, Foote RL, Schiff D, Gorman D, Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients J Neurosurg 90:42–50, 1999.
Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD, Gamma knife radiosurgery of image-diagnosed intracranial meningioma Int J Radiat Oncol Biol Phys 56(3): 801–806, 2003
Lee JYK, Niranjan A, McInerney J, Kondziolka D, Flickinger JC, Lundsford LD, Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas J Neurosurg 97: 65–72, 2002
Kyritisis AP, Chemotherapy for meningiomas J Neuro-Oncol 29: 269–272,1996
Grunberg SM, Weiss M, Lack of efficacy of megestrol acetate in the treatment of unresectable meningioma J Neuro-Oncol8:61–65, 1990
Grunberg SM, Weis M, Spitz IM, Ahmadi J, Sadun A, Russell CA, Lucci L, Stevenson LL, Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone J Neurosurg 74:861–866, 1991
Lamberts SWJ, Tanghe HLJ, Avezaata CJJ, Braakman R, Wijingaarde R, Koper JW, de Jong FH, Mifepristone (RU-486) treatment of meningiomas J Neurol Neurosurg Psych 55: 486–490, 1992
Grunberg SM, Rankin CR, Townsend J, Ahmadi J, Feun L, Fredricks R, Russell C, Kabbinavar F, Barger GR, Seltzer KJ, Phase III double blind randomized placebo-controlled study of mifepristone (RU-486) for the treatment of unresectable meningioma Proceedings ASCO 20: 56a, 2001 (abstract)
Wöber-Bringöl Ç, Wöber C, Marosi C, Prayer D, Interferon-alfa-2b for meningioma. Lancet 345: 331, 1995
Koper J, Zwarthoff E, Hagemeijer A, Braakman R, Avezaat CJ, Bergstrom M, Lamberts SW: Inhibition of the growth of cultured human meningioma cells by recombinant interferon-a. Eur J Cancer 27: 271–275, 1998
Kaba SE, DeMonte F, Bruner JM, et al, The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B Neurosurgery 40(2): 271–275, 1997
Schrell UMH, Ritting MG, Anders M, Kiesewetter F, Marschalek R, Koch UH, Fashlbusch R, Hydroxyurea for treatment of unresectable and recurrent meningiomas J Neurosurg 86:845–852, 1997
Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey L, Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma J Neurosurg 97: 341–346, 2002
Newton HB, Slivka MA, Stevens C, Hydroxyurea chemotherapy for unresectable or residual meningioma J Neuro-Oncol 49(2): 165–170, 2000
Chamberlain MC, Malignant meningiomas: adjunct combined modality therapy J Neurosurg 84: 733–736,1996
Chen TC: CPT-11 induces apoptosis in meningioma cells. Congress of Neurological Surgeons Annual Meeting Boston, MA, November 1999 (abstract)
Macdonald DR, Cascino TL, Schold Jr, SC, Cairncross JG, Response criteria for Phase II studies of supratentorial malignant glioma J Clin Oncol 8(7):1277–1280, 1990
Stupp R, Dietrich PY, Kraljevic SO, Picca A, Maillard I, et al, Promising survival for patients treated with newly diagnosed glioblastoma multiforme treated with concurrent radiation plus temozolomide followed by adjuvant temozolomide J Clin Oncol 20: 1375–1382, 2002
Hvizdos KM, Goa KL, Temozolomide CNS Drugs 12(3): 237–243, 1999
Acknowledgement
Ms. Nadine Stanton for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chamberlain, M.C., Tsao-Wei, D.D. & Groshen, S. Salvage chemotherapy With CPT-11 for recurrent meningioma. J Neurooncol 78, 271–276 (2006). https://doi.org/10.1007/s11060-005-9093-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-005-9093-x