Abstract
Purpose
This study aimed to compare the efficacy of topical bevacizumab and motesanib in an experimental corneal neovascularization model, and find the most effective motesanib dose.
Materials and methods
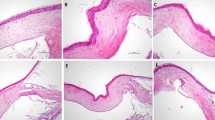
In experiments, 42 Wistar Albino rats were randomly divided into six groups (n = 7). Corneal cauterization was applied to all groups except the group 1. Group 1 did not receive any treatment. Topical dimethylsulfoxide was applied to sham group three times a day(tid). Topical bevacizumab drops (5 mg/ml) were applied to Group 3 tid. Topical motesanib drops with a dose of 2.5, 5, and 7.5 mg/ml were respectively applied in Groups 4, 5, and 6 tid. On the 8th day, corneal photographs of all rats were taken under general anesthesia, and the percentage of corneal neovascular area was calculated. VEGF-A mRNA, VEGFR-2 mRNA, miRNA-21, miRNA-27a, miRNA-31, miRNA-126, miRNA-184, and miRNA-204 were evaluated by the qRT-PCR method in corneas taken after decapitation.
Results
The percentage of corneal neovascularization areas and VEGF-A mRNA expression levels were decreased in all treatment groups compared to group 2 (p < 0.05). VEGFR-2 mRNA levels were found to be statistically significantly decreased in groups 4 and 6 compared to group 2 (p < 0.05). Statistically significant changes were detected in the expression levels of only miRNA-126 among all miRNAs.
Conclusion
Motesanib with a dose of 7.5 mg/ml statistically significantly suppressed the VEGFR-2 mRNA level compared with other treatment doses and may be more effective than bevacizumab. Further, miRNA-126 can be used as a proangiogenic marker.




Similar content being viewed by others
References
Zhang SX, Ma JX (2007) Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog Retin Eye Res 26:1–37
Feizi S, Azari AA, Safapour S (2017) Therapeutic approaches for corneal neovascularization. Eye and Vision 4:1–10
Philipp W, Speicher L, Humpel C (2000) Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Investig Ophthalmol Visual Sci 41:2514–2522
Ho QT, Kuo CJ (2007) Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol 39:1349–1357
Gan L, Fagerholm P, Palmblad J (2004) Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand 82:557–563
Scholl S, Kirchhof J, Augustin AJ (2010) Antivascular endothelial growth factors in anterior segment diseases. Anti-VEGF. Karger Publishers, vol 46, pp 133–139
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854
Zhang Y, Cai S, Jia Y, Qi C, Sun J, Zhang H et al (2017) Decoding noncoding RNAs: role of microRNAs and long noncoding RNAs in ocular neovascularization. Theranostics 7:3155–3167
Rodrigues EB, Farah ME, Maia M, Penha FM, Regatieri C, Melo GB et al (2009) Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res 28(2):117–144
Montanino A, Manzo A, Carillio G, Palumbo G, Esposito G et al (2021) Angiogenesis inhibitors in small cell lung cancer. Front Oncol 11:655316
Li C, Kuchimanchi M, Hickman D, Poppe L, Hayashi M, Zhou Y et al (2009) In vitro metabolism of the novel, highly selective oral angiogenesis inhibitor motesanib diphosphate in preclinical species and in humans. Drug Metab Dispos 37:1378–1394
Sawaki A, Yamada Y, Komatsu Y, Kanda T, Koseki M, Baba H et al (2010) Phase II study of motesanib in Japanese patients with advanced gastrointestinal stromal tumors with prior exposure to imatinib mesylate. Cancer Chemother Pharmacol 65:961–967
Sherman SI, Wirth LJ, Droz J-P, Hofmann M, Bastholt L, Martins RG et al (2008) Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 359:31–42
Tektemur A, Etem Önalan E, Kaya Tektemur N, Dayan Cinkara S, Kılınçlı Çetin A, Tekedereli et al (2021) Carbamazepine-induced sperm disorders can be associated with the altered expressions of testicular KCNJ11/miR-let-7a and spermatozoal CFTR/miR-27a. Andrologia 53:e13954
Prasadam I, Zhou Y, Du Z, Chen J, Crawford R, Xiao Y (2014) Osteocyte-induced angiogenesis via VEGF–MAPK-dependent pathways in endothelial cells. Mol Cell Biochem 386:15–25
Kamanu TK, Radovanovic A, Archer JA, Bajic VB (2013) Exploration of miRNA families for hypotheses generation. Sci Rep 3:1–8
Mukwaya A, Jensen L, Peebo B, Lagali N (2019) MicroRNAs in the cornea: role and implications for treatment of corneal neovascularization. Ocular Surface 17:400–411
Wang L, Lee AYW, Wigg JP, Peshavariya H, Liu P, Zhang H (2016) miR-126 regulation of angiogenesis in age-related macular degeneration in CNV mouse model. Int J Mol Sci 17:895
Bai Y, Bai X, Wang Z, Zhang X, Ruan C, Miao J, (2011) MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Exp Mol Pathol 91:471–477
Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ et al (2008) Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development 135:3989–39893
Yaşar M, Çakmak H, Dündar S, Örenay Boyacıoğlu S, Çalışkan M, Ergin K (2020) The role of microRNAs in corneal neovascularization and its relation to VEGF. Cutan Ocul Toxicol 39(4):341–347
Liu C-H, Huang S, Britton WR, Chen J (2020) MicroRNAs in vascular eye Diseases. Int J Mol Sci 21:649
Zhang Y, Zhang T, Ma X, Zou J (2017) Subconjunctival injection of antagomir-21 alleviates corneal neovascularization in a mouse model of alkali-burned cornea. Oncotarget 8:11797–11808
Urbich C, Kaluza D, Frömel T, Knau A, Bennewitz K, Boon RA et al (2012) MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood J Am Soc Hematol 119:1607–1616
Chen P, Yin H, Wang Y, Wang Y, Xie L (2012) Inhibition of VEGF expression and corneal neovascularization by shRNA targeting HIF-1α in a mouse model of closed eye contact lens wear. Mol Vis 18:864–873
Zhang X, Di G, Dong M, Qu M, Zhao X, Duan H et al (2018) Epithelium-derived miR-204 inhibits corneal neovascularization. Exp Eye Res 167:122–127
Zong R, Zhou T, Lin Z, Bao X, Xiu Y, Chen Y et al (2016) Down-regulation of MicroRNA-184 is associated with corneal neovascularization. Invest Ophthalmol Vis Sci 57(3):1398–1407
Al-Debasi T, Al-Bekairy A, Al-Katheri A, Al Harbi S, Mansour M (2017) Topical versus subconjunctival anti-vascular endothelial growth factor therapy (Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization. Saudi J Ophthalmol 31:99–105
Habot-Wilner Z, Barequet IS, MoisseievJ, Rossner M IY (2010) The inhibitory effect of different concentrations of topical bevacizumab on corneal neovascularization. Acta Ophthalmol 88:862–867
Zhang J, Yang PL, Gray NS (2009) Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 9:28–39
Pawson T (2002) Regulation and targets of receptor tyrosine kinases. Eur J Cancer 38:3–10
Yildirim H, Aydemir O, Balbaba M, Özercan IH, Ilhan N (2020) Comparison of the effect of topical bevacizumab and sorafenib in experimental corneal neovascularization. Cutan Ocul Toxicol 39:223–228
Sahan B, Ciftci F, Eyuboglu S, Yilmaz B, Yalvac BI (2019) Comparison of the effects of dovitinib and bevacizumab on reducing neovascularization in an experimental rat corneal neovascularization model. Cornea 38:1161–1168
Cakmak H, Gokmen E, Bozkurt G, Kocaturk T, Ergin K (2018) Effects of sunitinib and bevacizumab on VEGF and miRNA levels on corneal neovascularization. Cutan Ocul Toxicol 37(2):191–195
Rho CR, Kang S, Park KC, Yang KJ, Choi H, Cho WK (2015) Antiangiogenic effects of topically administered multiple kinase inhibitor, motesanib (AMG 706), on experimental choroidal neovascularization in mice. J Ocul Pharmacol Ther 31:25–31
Polverino A, Coxon A, Starnes C, Diaz Z, DeMelfi T, Wang L et al (2006) AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Can Res 66:8715–8721
Coxon A, Bready J, Kaufman S, Estrada J, Osgood T, Canon J (2012) Anti-tumor activity of motesanib in a medullary thyroid cancer model. J Endocrinol Invest 35:181–190
Coxon A, Bush T, Saffran D, Kaufman S, Belmontes B, Rex K et al (2009) Broad antitumor activity in breast cancer xenografts by motesanib, a highly selective, oral inhibitor of vascular endothelial growth factor, platelet-derived growth factor, and Kit receptors. Clin Cancer Res 15:110–118
Tebbutt N, Kotasek D, Burris HA, Schwartzberg LS, Hurwitz H, Golstein SJ, D, (2015) Motesanib with or without panitumumab plus FOLFIRI or FOLFOX for the treatment of metastatic colorectal cancer. Cancer Chemother Pharmacol 75:993–1004
Dell S, Peters S, Müther P, Kociok N, Joussen AM (2006) The role of PDGF receptor inhibitors and PI3-kinase signaling in the pathogenesis of corneal neovascularization. Invest Ophthalmol Vis Sci 47:1928–1937
Funding
Financial support was received from Fırat University Scientific Research Project Center (FÜBAP) for this submission (Project no: TF: 2021/028).
Author information
Authors and Affiliations
Contributions
Mukaddes Çelenk; concept, design, intellectuel content and drafting article. Hakan Yıldırım; design, concept and statistical analysis. Ahmet Tektemur; acquisition data, drafting article, interpretation of data. Mehmet Balbaba; biochemical analysis, interpretation of data. Murat Erdağ (corresponding author); acquisition of data, interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Animal research
This study was carried out in accordance with the ARVO Statement of Ophthalmic and Vision Research on Animal Use. The Fırat University Experimental Animal Studies Ethics Committee approved the study protocols.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çelenk, M., Yıldırım, H., Tektemur, A. et al. Effect of topical motesanib in experimental corneal neovascularization model. Int Ophthalmol 43, 2989–2997 (2023). https://doi.org/10.1007/s10792-023-02685-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02685-3