Abstract
Background and Aim
The controlled attenuation parameter (CAP) using transient elastography (TE) was validated in chronic hepatitis C to evaluate hepatic steatosis; however, limited data are available on chronic hepatitis B (CHB). Therefore, we assessed the accuracy and the efficacy of CAP for the detection of steatosis in CHB.
Methods
Consecutive CHB patients underwent liver biopsy and liver stiffness measurements (LSM) with simultaneous CAP determination using the M probe of the TE. The area under the receiver operating characteristics curve (AUROC) was used to evaluate the performance of CAP in diagnosing steatosis compared with biopsy.
Results
A total of 340 patients were included: 60 % were male, the median age was 37 years; the body mass index (BMI) was ≥28 kg/m2 for 14 % of the subjects; and the distribution of the steatosis grade was S0 58.2 %, S1 34.2 %, S2 5.0 % and S3 2.6 %. The median (range) of CAP was 218 (100–400) dB/m, and CAP correlated with the BMI (ρ = 3.622) and steatosis grade (ρ = 29.203) according to a multivariate analysis (both P < 0.001). CAP could detect the different grades of steatosis: ≥S1 with AUROC of 0.81 at a cutoff of 224 dB/m, ≥S2 with AUROC of 0.90 at a cutoff of 236 dB/m and ≥S3 with AUROC of 0.97 at a cutoff of 285 dB/m. Furthermore, the LSM and fibrosis and activity grades on biopsy did not influence the CAP performance.
Conclusions
CAP presented excellent diagnostic performance for severe steatosis with high sensitivity and specificity in Chinese patients with CHB.
Similar content being viewed by others
Introduction
Hepatic steatosis results from the accumulation of lipids in hepatocytes, which is common in patients with nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD) and chronic hepatitis C virus infection (CHC) [1]. NAFLD occurs in approximately one-third of the general population in the USA [2]. The prevalence of hepatic steatosis in patients with chronic hepatitis B virus infection (CHB) varies from 20 to 55 % [3–6]. CHB remains a major cause of liver-related morbidity and mortality, particularly in China [7, 8]. Coexistent steatosis in patients with CHC has been widely studied [9, 10]. Unlike CHC, several studies have indicated that metabolic factors rather than viral factors are responsible for the fat deposition in the liver in CHB patients [11, 12]. Although bland steatosis in NAFLD is considered to have a relatively benign prognosis [13], the coexistence of steatosis in patients with CHB is highly prevalent and may adversely affects the antiviral treatment [12, 14] and might even increase the risk of liver cirrhosis and hepatocellular carcinoma (HCC). Therefore, the detection of hepatic steatosis is essential in patients with chronic liver diseases. Nevertheless, studies on the coexistence of steatosis and CHB are much rare compared to studies of CHC, despite the high prevalence of CHB in China [7, 12].
Liver biopsy is regarded as the gold standard for the assessment of hepatic steatosis and liver fibrosis. However, it is limited by its invasiveness, cost and albeit uncommon, side effect profile, with reported serious complications occurring in 1–5 % patients [15]. Specifically, sampling errors may exist, as a biopsy represents only a tiny part of the whole liver [16]. Many imaging methods to assess hepatic steatosis or fibrosis have been developed over the past decades, including ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and proton magnetic resonance spectroscopy (MRS) [17]. However, the utility of these noninvasive means is limited by various concerns, such as the operator-dependent nature of ultrasound, the radiation of CT, the high cost of MRI and MRS and the controversial performance of the Steato test [18–20].
Recently, a noninvasive and low-cost method to detect hepatic steatosis has been developed to overcome these limitations. This novel physical parameter, known as the controlled attenuation parameter (CAP), is measured on the vibration-controlled transient elastography machine (Fibroscan, Echosens, Paris, France) [21]. Fibroscan, an ultrasound-based vibration-controlled transient elastography (VCTE) device that performs liver stiffness measurements (LSM), was used to assess the liver elasticity related to liver fibrosis [22] performed well in the detection of fibrosis in CHB [23]. CAP is a measurement of ultrasound attenuation (go and return path) at 3.5 MHz using signals acquired by the M probe of Fibroscan. Recently, several studies have demonstrated its good diagnostic accuracy in detecting hepatic steatosis in patients with CHC, CHB and NAFLD [24–30]. However, the data on the diagnostic performance of CAP for large patients samples with CHB are limited. Thus, this study primarily aimed to evaluate the diagnostic performance of CAP in detecting hepatic steatosis in Chinese patients with CHB.
Materials and Methods
Study Population
Of all the patients who underwent both liver biopsy and Fibroscan at Tianjin No. 2 People’s Hospital, China between July 2012 and December 2013, those who were positive for hepatitis B surface antigen (HBsAg) for at least 6 months were prospectively enrolled in this study. The exclusion criteria were as follows:(1) coinfection of other viral hepatitis; (2) daily alcohol consumption ≥20 g in women and ≥30 g in men; (3) positive autoantibody above 1:160; (4) serum levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) that exceeded 10 times the upper limit of normal (ULN) or total bilirubin level exceeding 2.5 times of ULN; (5) presence of HCC; (6) immune suppressive treatment within 1 year; (7) an interval between biopsy and Fibroscan longer than 14 days; and (8) biopsy specimen of noncirrhotic patients <15 mm long, diameter <0.8 mm or containing <6 portal areas. The study protocol was approved by the Ethics Committee of Tianjin No. 2 People’s Hospital and conformed to the provisions of the Declaration of Helsinki.
Biochemical Parameters
Serum samples were collected on the same or 3 days before/after liver biopsy. The laboratory data included the serum ALT, AST, gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total bilirubin, fasting plasma glucose (FPG), total cholesterol (TC), triglyceride, HBsAg, hepatitis B e antigen (HBeAg), anti-HBe and HBV DNA level. The HBsAg and HBeAg of each included patient were measured using an electrochemiluminescence assay (Roche COBAS e 601, USA), and the HBV DNA was determined by quantitative PCR (Roche 480 light Cycler, PCR-384-LC480-W, USA).
Liver Biopsy
All patients underwent a percutaneous ultrasound-guided liver biopsy. Each liver specimen was fixed in formalin, embedded in paraffin and stained with hematoxylin–eosin, Masson trichrome and collagen. Two experienced hepatic pathologists (Shi and Liu) who were blinded to the clinical data independently reviewed the histological findings. In case of disagreements, they would discuss their findings until a consensus was reached. The Knodell histological activity index (HAI) was used to describe the hepatocellular necro-inflammatory activity [31]. The liver fibrosis was semiquantitatively assessed according to Ishak systems and was graded as stage 0–stage 6 [32]. The hepatic steatosis was graded from 0 to 3 based on the number of hepatocytes with steatosis in the biopsy: S0: <5 %; S1: 5–33 %; S2: 34–66 %; S3: >66 % [33].
Measurement of LSM and CAP
All patients were examined using the 3.5 MHz M probe, which measured the liver stiffness 25–65 mm underneath the skin surface [34]. The 3.5 MHz ultrasound transducer mounted on the axis of an electromechanical transducer transmitted a vibration of 50 Hz and induced an elastic shear wave that propagates through the underlying tissues [35]. The LSM was expressed as kPa, and the examination was considered reliable if 10 valid LSMs were obtained, the success rate exceeded 60 %, and the ratio of the interquartile range (IQR) to the median of 10 measurements (IQR/M) was <0.3 [19]. CAP is a novel proprietary algorithm performed simultaneously with LSM using the ultrasonic signals acquired by the Fibroscan to detect hepatic steatosis. CAP measures the ultrasound attenuation (go and return path) using signals acquired by the 3.5 MHz Fibroscan probe, which measured the same liver area measured by LSM. The CAP values were expressed as dB/m and are valid if the LSM is valid. The operator was blinded to the patients’ clinical and histological data.
Statistical Analysis
All data are presented as number (%) and median (range) values. A Mann–Whitney test (two groups comparison) or Kruskal–Wallis (more than two groups comparison) test was used for continuous variables. We evaluated the distribution of CAP values of each degree of steatosis and BMI using box plots. Kendall’s rank correlation coefficients were used for two ordinal variables, and a Spearman correlation was for two continuous or continuous and ordinal variables. Univariate linear regressions were used to assess the association of CAP with demographic, clinical and histological factors, and significant factors in the univariate analysis (P < 0.2) were included in the multivariate analysis using multiple linear regressions. The diagnostic efficiency of CAP and LSM was analyzed by computing the areas under the receiver operating characteristics curves (AUROC) and their 95 % confidence interval (CI). The optimal cutoff value, sensitivity, specificity, positive predictive value/negative predictive value (PPV/NPV) and positive likelihood ratio/negative likelihood ratio (PLR/NLR) were calculated by maximizing the Youden index (sensitivity + specificity − 1) to evaluate the diagnostic performance of CAP and LSM. The diagnostic efficiencies between two groups were compared using the Z test. Statistical analyses were performed on SPSS 15.0 (SPSS, Chicago, USA), MedCalc 9.3 (MedCalc Software, Mariakerke, Belgium) and OriginPro 9.0 (OriginPro, Northampton, USA).
Results
Clinical and Histological Characteristics of the Study Population
Between July 2012 and December 2013, a total of 444 individuals who underwent both liver biopsy and Fibroscan at Tianjin No. 2 People’s Hospital with HBsAg-positive tissue for at least 6 months were enrolled in our study. One hundred and four patients were excluded for the following reasons: (1) the presence of HCC (n = 89); (2) daily alcohol consumption ≥20 g in women and ≥30 g in men (n = 3); (3) positive autoantibody above 1:160 (n = 5); (4) serum ALT levels exceeding 10 times the ULN or total bilirubin levels exceeding 2.5 times the ULN (n = 5); (5) immunosuppressive treatment within 1 year (n = 1); and (6) biopsy specimen <15 mm long or a diameter of <0.8 mm (n = 1).
For the 340 patients considered in the subsequent analysis, the median age was 37 years old and 205 patients (60.3 %) were male. The distribution of the degree of hepatic steatosis was S0 (58.2 %, n = 198), S1 (34.2 %, n = 116), S2 (5.0 %, n = 17) and S3 (2.6 %, n = 9). The clinical and histological characteristics of the study population are shown in Table 1.
LSM for Assessment of Liver Fibrosis
The AUROCs, optimal cutoff points, sensitivity, specificity, PPV/NPV and PLR/NLR of LSM for liver fibrosis diagnostic are summarized in Table 2. The AUROCs for the detection of Ishak fibrosis ≥stage 2, ≥stage 3, ≥stage 4, ≥stage 5 and stage 6 were 0.672 (95 % CI 0.619–0.722), 0.813 (95 % CI 0.768–0.853), 0.790 (95 % CI 0.743–0.832), 0.805 (95 % CI 0.759–0.846) and 0.849 (95 % CI 0.806–0.885), respectively.
CAP for Assessment of Hepatic Steatosis
The median (range) of CAP of the study population was 217.5 (100–400) dB/m. The distributions of CAP for each degree of hepatic steatosis are presented in Fig. 1. The median CAP values of S0, S1, S2 and S3 were 197.0 (100–291), 239.5 (119–342), 276.0 (224–365) and 315 (260–400), respectively. In addition, the CAP values of S1 were significantly higher and lower than those of S0 (P < 0.001) and S2 (P = 0.002), respectively. Furthermore, the difference between S2 and S3 was at the limit of statistical significance (P = 0.063), and the differences in the CAP values among different fibrosis stages were insignificant (P > 0.05).
The distribution of CAP (dB/m) based on histological steatosis grade (S0, S1, S2 and S3). The CAP values of S1 were significantly higher and lower than those of S0 (P < 0.001) and S2 (P = 0.002), respectively, while the difference between S2 and S3 was at the limit of significance (P = 0.063); CAP controlled attenuation parameter
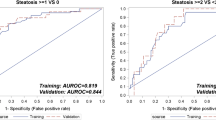
For the diagnosis of hepatic steatosis, CAP could predict the steatosis degree ≥S1 with a good AUROC [0.811 (95 % CI 0.765–0.851)], sensitivity (73.2 %) and specificity (75.8 %) when the optimal cutoff was at 224 dB/m. Moreover, CAP could detect the histological grade ≥S2 with an excellent AUROC [0.900 (95 % CI 0.863–0.930)], sensitivity (92.3 %) and specificity (70.4 %) when the optimal cutoff was at 236 dB/m. The AUROC for a steatosis grade of S3 was 0.973 (95 % CI 0.950–0.988), with a sensitivity of 100.0 % and specificity of 92.8 % when the optimal cutoff was at 285 dB/m. The AUROCs, optimal cutoff points, sensitivity, specificity, PPV/NPV and PLR/NLR of CAP for hepatic steatosis are outlined in Table 3 and Fig. 2. Moreover, the efficiency of CAP in differentiating two different steatosis grades was further evaluated between S0 and S1, S0 and S2, S0 and S3, S1 and S2, S1 and S3, and S2 and S3, and the results are outlined in Table 4.
The Association of CAP with Demographic, Biochemical, and Histological Factors
To observe the influence of demographic, biochemical and histological factors on CAP, we performed a univariate analysis that included the age, gender, BMI, ALT, AST, GGT, ALP, total bilirubin, FPG, TC, triglyceride, HBeAg-positive rate, Log 10 HBV DNA, hepatic steatosis grade, HAI and Ishak fibrosis grade. The age, BMI, ALT, total bilirubin, TC, triglyceride, hepatic steatosis grade and HAI significantly correlated with CAP (P < 0.2). However, only the BMI and steatosis grade still significantly correlated with CAP when adjusting for age and gender in a subsequent multivariate analysis (P < 0.001), as shown in Table 5. Moreover, the steatosis grade showed an insignificant relationship to the Ishak fibrosis staging and HAI in liver biopsy (P > 0.05).
Discussion
Despite the introduction of the HBV vaccine, viral hepatitis due to the hepatitis virus B (HBV) remains a major global health problem, especially in China [7, 36, 37]. The prevalence of CHB has previously been reported to be 3,500 million worldwide, with 93 million of these cases being in China (7.18 % of the general population) [7, 36, 37]. Because CHB infection is a major cause of cirrhosis and HCC, antiviral treatment is of paramount importance for CHB patients. However, hepatic steatosis has been raging in Chinese patients with CHB in the past decade [30], and it has been suggested to be significantly associated with antiviral therapy failure and may accelerate the disease progression [38, 39]. These phenomena might be due to the following reasons: First, hepatic steatosis is an important determinant of insulin resistance and closely associated with metabolic syndrome [40]. The accumulation of lipids in hepatocytes increases the risk of type 2 diabetes, hyperlipidemia, atherosclerosis and coronary heart disease, even leading to premature death [41]. Moreover, increased aminotransferase levels may result from NAFLD instead of the immune clearance of HBV, which may lead to the improper timing of antiviral therapy. Hepatic steatosis might be a common factor associated with elevation of serum aminotransferases in HBsAg-positive CHB patients with low HBV DNA loads, especially in patients treated with nucleoside analogs [42]. Therefore, the detection of hepatic steatosis is essential for CHB-infected patients to assess the efficacy of therapies and choose the appropriate timing of antiviral therapy. Although liver biopsy is still regarded as the gold standard to evaluate hepatic steatosis and fibrosis, it is limited by its invasiveness, cost, potential serious complications and possible sampling error. Therefore, a novel noninvasive method to assess hepatic steatosis, known as CAP, may be more appropriate for wide applications in clinical practice.
At the present study, the diagnostic performance of CAP in assessing hepatic steatosis in Chinese patients with CHB was excellent for steatosis grades ≥S1, ≥S2 and =S3 (all AUROCs >0.8). CAP appeared to highly accurately detect severe steatosis (>66 %) with a sensitivity and specificity that both exceeded 90 %. In addition, our study also reconfirmed a previous study that suggested that CAP was an ideal tool to screen for hepatic steatosis due to its high negative predictive value [24]. However, the AUROCs of this method (<0.8) indicated only a fair efficacy in discriminating between two contiguous steatosis grades, which was supported by several studies [24–30]. Specifically, the difference in CAP between S2 and S3 was at the limit of statistical significance (P = 0.063), which suggested that CAP might be suboptimal in differentiating moderate and severe steatosis. This shortcoming might be caused by the pathologist’s reading error between two consecutive stages and limited sample size of each steatosis grade. Consequently, studies that examine a large cohort of patients with CHB infection may be warranted in the future.
In this study, the optimal cutoff values of CAP for steatosis grades ≥S1, ≥S2 and =S3 were 224, 236 and 285 dB/m, respectively, which were close but separated. These values were shown to be much lower than those given in the study of Myers et al. [27], in which 153 patients with BMI >28 kg/m2 were included, and their cutoff values for each steatosis grade were 289, 288 and 283 dB/m, respectively. Higher cutoff values were also reported in several other studies with BMI values similar to those in our study [26, 29]. Because our cutoff values were in concordance with studies that assessed the mono-infection of HBV [24, 25], the spectrum bias and baseline difference in the BMI might contribute to the higher proportion (58.2 %) of patients without hepatic steatosis in our cohort, which further reduced the cutoff values. These findings need to be further validated. Two studies have shown that the accuracy of CAP did not significantly differ among various etiologies of liver disease [25, 30]. The diagnostic performance in our study was higher than that reported in a previous study of patients with CHB in India [25]. This difference might result from the lack of description regarding the double-blinding consensus reading of the pathologists in the Indian study.
There were several studies indicating that traditional reliable criteria of LSM measurements (at least 10 valid LSMs were obtained, success rate exceeded 60 %, and IQR/M was <0.3) should be re-scrutinized for the number of valid acquisitions and the success rate had no effects on the diagnostic accuracy [43–45]. However, the new set of reliability criteria could not be necessarily applied to our study because most of their included patients used CHC and METAVIR scoring system instead of Ishak for fibrosis. As a result, future studies on the reliability criteria of LSM measurements for patients with CHB were needed.
Our study also showed that CAP appeared to be significantly correlated with the BMI in a multivariate analysis. However, the influence of BMI on the CAP measurement was significantly smaller than that of hepatic steatosis in biopsy (ρ = 3.622, 29.203, respectively, in multivariate analysis). We have not set up different cutoffs of CAP for patients with different BMI level in this study. Actually, BMI was no longer the independent factors of CAP in patients with chronic liver diseases when the skin-liver capsule distance (SCD) was applied to multivariate analysis in our unpublished data. In contrast to normal SCD, a long SCD (>25 mm) consistently produced approximately 60 db/m larger CAP values at different grade of hepatic steatosis in patients with chronic liver diseases using the M probe (data not shown). The 3.5 MHz M probe can measure the liver stiffness 25–65 mm underneath the skin surface. When the SCD exceeds 25 mm, the XL probe is needed because the longer distance caused by the large amounts of subcutaneous fat of overweight or obese individuals may affect the shear wave and measurements [22, 43]. However, CAP is not yet available for the XL probe. As a result, BMI might be a confounding factor of subcutaneous adiposity.
CAP on Fibroscan presents many advantages according to the current study. First, it is noninvasive and painless with reproducible LSM and CAP values simultaneously. Unlike well known in the literature that AUROC increases with fibrosis stage [22, 35, 42], the AUROC of LSM for detecting fibrosis stage 3–6, stage 4–6 and stage 5–6 was similar in our study. Actually, all the AUROCs of LSM for fibrosis stage 3–6, stage 4–6 and stage 5–6 were higher and lower than that in fibrosis stage 2–6 and in stage 6, respectively. Second, CAP can evaluate a liver parenchyma volume 100 times larger than a liver biopsy sample and thus is more representative of the liver than biopsy [22, 35]. Finally, our study indicated that CAP correlated with the hepatic steatosis grade independent of the HAI and liver fibrosis stage and was efficient in diagnosing steatosis in HBV-infected Chinese patients.
This study also suffered from several limitations. First, the sample size of our study was limited which resulted in the less proportion of moderate and severe steatosis or Ishak stage 5 and 6. Thus, a large cohort of patients with CHB is needed to corroborate the diagnostic efficiency of CAP. Second, we did not evaluate the influence of waist circumstance and SCD on CAP measurement. Whether SCD or waist circumstance truly affects CAP values should be explored in a future study. Finally, the combination of CAP and another noninvasive method to detect hepatic steatosis should be studied to improve the diagnostic performance or yield a high PPV or NPV for different applications in clinical practice. In addition, CAP was not correlated with serum elevation of ALT in CHB patients at the present study. Thus, the role of CAP measurement in assisting the decision-making process for treatment of hepatitis B in a patient who has coexistence of CHB and hepatic steatosis is still not clear.
In conclusion, CAP measured on Fibroscan, as a novel physical parameter, presented excellent diagnostic performance for severe steatosis (>66 %) with high sensitivity and specificity among Chinese patients with CHB. In addition, CAP was also an ideal tool to screen for hepatic steatosis among Chinese patients with CHB due to its high negative predictive value.
References
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases. Am Coll Gastroenterol Assoc Hepatol. 2012;35:2005–2023.
Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395.
Czaja AJ, Carpenter HA. Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology. 1993;105:1824–1832.
Bondini S, Kallman J, Wheeler A, et al. Impact of non-alcoholic fatty liver disease on chronic hepatitis B. Liver Int. 2007;27:607–611.
Thomopoulos KC, Arvaniti V, Tsamantas AC, et al. Prevalence of liver steatosis in patients with chronic hepatitis B: a study of associated factors and of relationship with fibrosis. Eur J Gastroenterol Hepatol. 2006;18:233–237.
Wang CC, Hsu CS, Liu CJ, Kao JH, Chen DS. Association of chronic hepatitis B virus infection with insulin resistance and hepatic steatosis. J Gastroenterol Hepatol. 2008;23:779–782.
Chan HL, Jia J. Chronic hepatitis B in Asia-new insights from the past decade. J Gastroenterol Hepatol. 2011;26:131–137.
Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28:11–17.
Poynard T, Ratziu V, McHutchison J, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75–85.
Harrison SA, Brunt EM, Qazi RA, et al. Effect of significant histologic steatosis or steatohepatitis on response to antiviral therapy in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2005;3:604–609.
Altlparmak E, Koklu S, Yalinkilic M, et al. Viral and host causes of fatty liver in chronic hepatitis B. World J Gastroenterol. 2005;11:3056–3059.
Wang MM, Wang GS, Shen F, Chen GY, Pan Q, Fan JG. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors. Dig Dis Sci. (Epub ahead of print). doi:10.1007/s10620-014-3180-9.
Lesmana LA, Lesmana CR, Pakasi LS, Krisnuhoni E. Prevalence of hepatic steatosis in chronic hepatitis B patients and its association with disease severity. Acta Med Indones. 2012;44:35–39.
Jin X, Chen YP, Yang YD, Li YM, Zheng L, Xu CQ. Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS One. 2012;7:e34198.
Maharaj B, Maharaj RJ, Leary WP, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525.
Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906.
Lee SS, Park SH, Kim HJ, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579–585.
Cesbron-Métivier E, Roullier V, Boursier J, et al. Noninvasive liver steatosis quantification using MRI techniques combined with blood markers. Eur J Gastroenterol Hepatol. 2010;22:973–982.
Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281–285.
Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487–495.
Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835.
Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713.
Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242–247.
Sasso M, Tengher-Barna I, Ziol M, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan (R): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244–253.
Kumar M, Rastogi A, Singh T, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013;28:1194–1201.
Chon YE, Jung KS, Kim SU, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102–109.
Myers RP, Pollett A, Kirsch R, et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–910.
Masaki K, Takaki S, Hyogo H, et al. Utility of controlled attenuation parameter (CAP) measurement for assessing liver steatosis in Japanese patients with chronic liver diseases. Hepatol Res. 2013;. doi:10.1111/hepr.12094.
de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918.
Shen F, Zheng RD, Mi YQ, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis in Chinese patients. World J Gastroenterol. 2014;20:4702–4711.
Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435.
Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699.
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474.
Sandrin L, Tanter M, Gennisson JL, Catheline S, Fink M. Shear elasticity probe for soft tissues with 1-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:436–446.
Wong GL. Transient elastography: kill two birds with one stone? World J Hepatol. 2013;5:264–274.
Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38:S158–S168.
Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China-declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–6557.
Negro F, Clement S. Impact of obesity, steatosis and insulin resistance on progression and response to therapy of hepatitis C. J Viral Hepat. 2009;16:681–688.
Ates F, Yalniz M, Alan S. Impact of liver steatosis on response to pegylated interferon therapy in patients with chronic hepatitis B. World J Gastroenterol. 2011;17:4517–4522.
Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2012;55:2005–2023.
Shi JP, Fan JG, Lou GQ, et al. Hepatic steatosis: a common reason for elevated alanine aminotransferase levels in HBsAg-positive chronic hepatitis B patients with low HBV DNA loads]. Zhonghua Gan Zang Bing Za Zhi. 2008;16:818–822.
Wong VW, Wong GL, Chan FK. Liver stiffness measurement: simplicity is prerequisite for reliability. Gastroenterology. 2013;144:243–244.
Boursier J, Zarski JP, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology.. 2013;57:1182–1191.
Lucidarme D, Foucher J, Le Bail B, et al. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1083–1089.
Acknowledgments
We wish to thank all the patients and medical staff who participated in the study. This work was financially supported by the State Key Development Program for Basic Research of China (2012CB517501), the Chinese Foundation for Hepatitis Prevention and Control—“Wang Bao-en liver fibrosis research fund (XJS20120501)” and the Science and Technology Foundation of Tianjin Municipal Health Bureau (12KG119).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mi, YQ., Shi, QY., Xu, L. et al. Controlled Attenuation Parameter for Noninvasive Assessment of Hepatic Steatosis Using Fibroscan®: Validation in Chronic Hepatitis B. Dig Dis Sci 60, 243–251 (2015). https://doi.org/10.1007/s10620-014-3341-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3341-x