Abstract
The aim of this study was to compare clinical cure rate, recurrence rate and time to resolution of diarrhea in patients with severe and severe-complicated Clostridium difficile infection (CDI) treated with teicoplanin or vancomycin. This two-year prospective observational study included patients with first episode or first recurrence of CDI who had severe or severe-complicated CDI and were treated with teicoplanin or vancomycin. Primary outcomes of interest were clinical cure rate at discharge and recurrence rate after eight weeks follow up, and secondary outcomes were all-cause mortality and time to resolution of diarrhea. Among 287 study patients, 107 were treated with teicoplanin and 180 with vancomycin. The mean age of patients was 73.5 ± 10.6 years. One hundred eighty six patients (64.8%) had prior CDI episode. Severe complicated disease was detected in 23/107 (21.5%) and 42/180 (23.3%) patients treated with teicoplanin and vancomycin, respectively. There was no statistically significant difference in time to resolution of diarrhea between two treatment arms (6.0 ± 3.4 vs 6.2 ± 3.1 days, p = 0.672). Treatment with teicoplanin resulted in significantly higher clinical cure rate compared to vancomycin [90.7% vs 79.4%, p = 0.013, odds ratio (OR) (95% confidence interval (CI)) 2.51 (1.19–5.28)]. Recurrence rates were significantly lower in patients treated with teicoplanin [9/97 (9.3%) vs 49/143 (34.3%), p < 0.001, OR (95%CI) 0.20 (0.09–0.42)]. There was no statistically significant difference in overall mortality rate. Teicoplanin might be a good treatment option for patients with severe CDI. Patients treated with teicoplanin experienced remarkably lower recurrence rates compared to vancomycin-treated patients.
Similar content being viewed by others
Introduction
Severe Clostridium difficile infection (CDI) is an important cause of mortality and morbidity in hospitalized patients, especially among elderly and those with multiple comorbidities [1, 2]. High recurrence rates following standard therapy are one of the major issues in the CDI management. Recurrent disease is not only an economic healthcare burden, but it also interferes with patients’ recovery from the disease preceding CDI, affects the quality of life and increases the risk of other hospital-acquired infections, CDI-related complications and unfavorable outcome [3]. In addition, after discharge, patients with recurrent disease become the reservoir for C. difficile spread outside hospital settings, even after successful treatment [3]. Although the introduction of fidaxomicin has led to lower recurrence rates in comparison to vancomycin and metronidazole, these rates remained notable, especially among patients with prior CDI and/or severe disease [4, 5].
The non-inferiority of oral teicoplanin to oral vancomycin in the CDI treatment with lower recurrence rates has already been demonstrated [6, 7]. However, these studies were performed on small numbers of patients and in the pre-epidemic era, before the emergence of new, hypervirulent strain, NAP1/BI/027 [6, 7]. From 2013, teicoplanin was approved for the CDI treatment [8].
Even though treatment of severe disease represents one of the major challenges in CDI due to high recurrence and mortality rates, only few real-life studies have addressed this issue.
We previously demonstrated the efficacy of oral teicoplanin in the treatment of severe and complicated CDI refractory to standard therapy [9]. Since these results were obtained from a small cohort, a large-scale study was needed.
The aim of this study was to assess and compare clinical cure rate, recurrence rate and time to resolution of diarrhea in patients with severe and severe complicated CDI treated with teicoplanin or vancomycin.
Methods
Study design and study population
This two-year prospective observational study was conducted in the University Hospital for Infectious and Tropical Disease, Clinical Centre of Serbia in Belgrade, from 1st August 2013 until 31st July 2015. Patients were recruited among those admitted to hospital for treatment of confirmed or suspected CDI. The study included patients with first episode or first recurrence of confirmed CDI who had severe or severe and complicated forms of the disease. Patients with life-threatening disease on admission were not included in the analysis in order to avoid selection bias, because all of them were given vancomycin on admission due to previous deficient and outdated experience about teicoplanin efficacy in these patients. Patients with concomitant bowel diseases (malignancy, inflammatory bowel diseases or any kind of enteropathy) were excluded from the study since these conditions could per se be associated with diarrhea. Those with second or subsequent recurrence were also excluded due to different treatment approaches they require.
Upon admission, patients with severe CDI were given oral vancomycin 125 mg q6h or oral teicoplanin 100 mg q12h and were treated for at least ten days. Those with severe complicated CDI initially received intravenous metronidazole 500 mg q8h in combination with either oral vancomycin 500 mg q6h or oral teicoplanin 200 mg q12h. Upon the initiation of the study vancomycin and teicoplanin were given to patients alternately (first patient who fulfilled our inclusion criteria received vancomycin, the second was given teicoplanin, and so on...). We chose to allocate drugs alternately in order to minimize selection bias. But, during the study period, one or the other drug was sporadically not available. During those time points all patients who were about to enter the study were given the only drug which was available at that moment (vancomycin or teicoplanin). It is worth mentioning, that the drug which was given to the patient at the beginning of therapy was always provided for the full course of 14-day treatment right upon entering the study, so that in case of potential later absence of certain drugs for new patients, patients who had already started one treatment regimen could fulfill their course of therapy without the need to switch to another drug. Patients were given vials orally for parenteral use because oral formulation of neither teicoplanin nor vancomycin was available in Serbia. Vials were reconstituted with sterile water for injection in the hospital pharmacy and stored at 2–8 °C for the maximum of 24 h. When administered by oral route teicoplanin is not absorbed from the gastrointestinal tract, therefore there is no risk of systemic adverse effects and no need for dosage adjustment in patients with renal failure [10]. In patients with ileus or toxic megacolon, vancomycin or teicoplanin enema was given in addition to combination therapy. The standard enema dosages were 500 mg of vancomycin and 200 mg of teicoplanin dissolved in 150 ml 0.9% normal saline, applied rectally, q6h and q12h, respectively. Primary outcomes of interest were clinical cure rate after 14 days of treatment and recurrence rate after eight weeks follow up, and secondary outcomes were all-cause mortality at the end of treatment and time to resolution of diarrhea.
Criteria for diagnosis of CDI and its severity
The diagnosis of CDI was confirmed in patients with diarrhea, in case of both positive enzyme immunoassay (EIA) test for detection of C. difficile toxin A and B in stool [RIDA®QUICK Clostridium difficile Toxin A/B (R-Biopharm AG, Darmstadt, Germany)] and positive stool culture (medium Clo Agar, BioMerieux, 69,280, Marcy l’Etoile, France) or in case of endoscopic findings of pseudomembranous colitis. Stool specimens were collected in sterile containers and sent to the hospital microbiology laboratory where they were processed within 24 h. The stool samples of all patients with clinical suspicion and the presence of risk factors for CDI were analyzed for the presence of C. difficile toxins A and B and cultivation of all samples for C. difficile was also performed. Tests for toxin detection were performed upon admission of the patient and the results were obtained after a couple of hours. All patients with positive toxins were initially considered to suffer from CDI, and were included in the study in case of fulfilling other inclusion criteria. After the results of culture were obtained, patients with positive toxins and positive C. difficile culture were confirmed to have CDI. For those patients who had negative C. difficile culture but had positive toxins, rectosigmoidoscopy or colonoscopy was performed in order to rule out patients with false positive test results for C. difficile toxins A and B, and in all of them typical pseudomembranes were found, thus confirming the diagnosis of pseudomembranous colitis. There were no patients with positive C. difficile toxins, and negative C. difficile culture who did not have pseudomembranes on colonic mucosa when endoscopy was performed. In case of negative tests for detection of C. difficile toxins A and B, culture was also performed, but because of the fact that the results of culture are obtained after a few days, all patients with high clinical and epidemiological suspicion of CDI and negative results of tests for C. difficile toxin A and B also underwent endoscopy and those with the findings of pseudomembranous colitis (and without other concomitant bowel diseases) were included in the study.
Severe CDI was diagnosed in the presence of endoscopic evidence of pseudomembranous colitis, or two or more of the following criteria: leucocyte count of above 15,000 cells/mm3 on admission, increased serum creatinine level (at least 1.5 times the premorbid level), hypoalbuminaemia (< 30 g/l) and fever ≥38.5 °C. Severe and complicated CDI was defined as the presence of ileus, toxic megacolon, hypotension requiring the use of vasopressors, and/or end organ failure (either renal or respiratory failure). Mild-to-moderate CDI was defined as the presence of diarrhea plus any additional sign or symptom not meeting severe or complicated criteria. Patients with refractory septic shock, severe consciousness impairment (stupor or coma) not explained by other causes except CDI, as well as those requiring ventilator support were considered to suffer a life-threatening disease.
Diarrhea was defined as three or more unformed stools per day, and time-to-resolution of diarrhea as the time from the therapy initiation to less than three unformed stools per day [8]. We defined clinical cure as resolution of diarrhea without its reappearance during treatment, and recurrence as the reappearance of diarrhea in clinically cured patients within eight weeks after therapy was completed. Treatment failure was determined as persistence of diarrhea after 14 days of treatment and the need to add or switch to another treatment option.
Data collection
Data about patients’ clinical and demographic characteristics, comorbidity, as well as their baseline laboratory findings were recorded on admission. Patients’ comorbid conditions were presented using Charlson comorbidity index (CCI) [11]. Data regarding the course of illness during hospital stay, the usage of concomitant antibiotics, and time to resolution of diarrhea were collected by daily examination and interview with patients, by reviewing medical records and by reports of the nurse in charge. The disease outcome at the end of therapy was also recorded. Clinically cured patients were followed for eight weeks after the treatment was completed by regular check-ups scheduled in two-week intervals in order to record possible recurrences. The follow-up period of eight weeks was chosen because according to current guidelines, the reappearance of diarrhea during this period is considered to be relapse, and after that reinfection. Patients were also advised to come to the hospital any time between check-ups, in case of diarrhea reappearance.
Ethics
The ethical committee of the Clinical Centre of Serbia approved the study (approval No 25/2013). Patients (or caregivers) signed a written, informed consent. The study was performed according to the declaration of Helsinki and subsequent revisions [12].
Statistical analysis
All data were analyzed using the methods of descriptive and analytic statistics. Univariate analysis was performed using Student’s t-test for continuous and chi-square and Fisher’s exact test for categorical variables. In order to evaluate the independent risk factors for recurrence, binary logistic regression analysis was performed. Variables that were significant in univariate analysis as well as those that had a priori clinical significance were included in the logistic regression analysis. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. The probability of diarrhea resolution and recurrence in two treatment groups was estimated using Kaplan-Meier survival analysis and compared by log rank test. Statistical Package for the Social Sciences (SPSS) software for Windows (version 17.0) was used for statistical analysis. Statistical significance was set at 0.05.
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Results
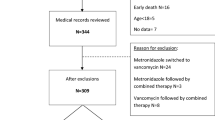
During the study period, 585 patients with confirmed CDI were treated in the Gastroenterology Department of the University Hospital for Infectious and Tropical Diseases, Clinical Centre of Serbia. Among them, 319 patients were eligible for the study, after we had excluded those with mild to moderate form of CDI (96 patients), directly life-threatening disease (58), second or subsequent recurrence (48), and patients with concomitant bowel diseases (64). Thirty-two patients dropped out during follow up, so we finally analyzed 287 patients, of which 107 were treated with teicoplanin and 180 with vancomycin.
Eighty-four (78.5%) patients treated with teicoplanin and 138 (76.7%) treated with vancomycin had severe non-complicated CDI, while complicated CDI was present in 23 (21.5%) and 42 (23.3%) patients treated with teicoplanin and vancomycin, respectively.
The mean age of patients was 73.5 ± 10.6 years (Table 1). One hundred eighty six patients (64.8%) had prior CDI episode. These and other clinical and demographic characteristics of patients, as well as their comorbidity and baseline laboratory analyses are shown in Table 1.
Previous surgery was more frequent in patients treated with teicoplanin [p = 0.005, OR (95% CI) 0.49 (0.30–0.79)]. The two treatment groups did not differ significantly with respect to any other of the baseline characteristics (Table 1).
Figure 1 represents a Kaplan-Meier analysis of time to resolution of diarrhea following the initiation of therapy according to different treatment regimens.
Kaplan-Meier analysis of estimated median time to resolution of diarrhea in patients with severe and severe-complicated Clostridum difficile infection treated with teicoplanin and vancomycin. Day 0 is defined as the day of therapy initiation. Estimated median time to resolution of diarrhea was 5 days (95% CI 4–6 days) in patients treated with vancomycin and 5 days (95% CI 4–6 days) in those treated with teicoplanin. There was no difference in the estimated time to diarrhea resolution between two treatment groups (p = 0.300)
The mean time to resolution of diarrhea was 6.1 ± 3.2 days. There was no statistically significant difference in time to resolution of diarrhea among patients treated with teicoplanin and vancomycin (6.0 ± 3.4 vs 6.2 ± 3.1 days, p = 0.672). Complicated CDI and the use of concomitant antibiotic therapy, but not recurrent disease, were significantly associated with longer time to resolution of diarrhea (7.5 ± 3.8 vs 5.8 ± 2.9 days, 8.7 ± 4.5 vs 5.6 ± 2.5 days, and 6.4 ± 3.4 vs 5.7 ± 2.9 days; p < 0.001, p < 0.001, and p = 0.065, respectively).
Treatment with teicoplanin resulted in significantly higher overall clinical cure rate than did treatment with vancomycin [90.7% vs 79.4%, p = 0.013, OR 2.51 (1.19–5.28)]. Teicoplanin-treated patients achieved higher clinical cure rates in each of the severity categories but this difference did not reach statistical significance (p = 0.062 and p = 0.184, for non-complicated and complicated CDI, respectively) (Table 2).
Recurrence rates following successful therapy were significantly lower in patients treated with teicoplanin compared to vancomycin-treated patients [9/97 (9.3%) vs 49/143 (34.3%), p < 0.001, OR (95%CI) 0.20 (0.09–0.42)]. When compared according to the disease severity, recurrence rates in severe CDI were 8/80 (10.0%) and 42/120 (35.0%), and in severe complicated CDI 1/17 (5.9%) and 7/23 (30.4%) in patients treated with teicoplanin and vancomycin, respectively [p < 0.001, OR (95%CI) 0.21 (0.09–0.47), and p = 0.107, OR (95%CI) 0.14 (0.02–1.30)]. Binary logistic regression analysis showed that the use of teicoplanin was an independent predictor of lower risk for recurrence after adjustment for confounders (Table 3).
Teicoplanin treatment also resulted in significantly lower recurrence rate in comparison to vancomycin among patients over 65 years old [6/66 (9.1%) vs 42/113 (37.2%), p < 0.001, OR (95%CI) 0.15 (0.06–0.39)]. Among those with complicated CDI and patients who needed concomitant antibiotic therapy, recurrence rates were lower in the teicoplanin treatment group, but without statistical significance [1/17 (5.9%) vs 7/23 (30.4%), p = 0.107, OR 0.14 (0.02–1.30), and 2/20 (10.0%) vs 4/23 (17.4%), p = 1.000, OR (95%CI) 0.64 (0.11–3.89), respectively].
The mean time to recurrence was 13.9 ± 12.3 days, and it was similar between patients treated with vancomycin and teicoplanin (14.0 ± 12.9 vs 13.2 ± 8.7 days, p = 0.829). Figure 2 represents Kaplan-Meier analysis of estimated median time to recurrence after achieved clinical cure showing no difference between two treatment arms.
Kaplan-Meier analysis of estimated median time to recurrence in patients with severe and severe complicated Clostridum difficile infection treated with teicoplanin and vancomycin. Day 0 is defined as the day the therapy was completed. The estimated median time to recurrence was 10 days (95% CI 8–13 days) in vancomycin-treated patients, and 10 days (95% CI 2–18 days) in those treated with teicoplanin. There was no difference in time to recurrence between two treatment groups (p = 0.874)
Discussion
During the last 15 years, the incidence of CDI has significantly increased, with more patients experiencing severe and recurrent disease.
CDI usually affects older patients and those with multiple comorbidities [1, 2]. In the United States a total of 93% of deaths from CDI and 92% of CDI-related hospitalization occurred in patients over 65 years old [13]. The mean age of patients in the present study was 73.5 ± 10.6 years, and patients over 65 years old accounted for 78% of the study population. Older patients have an increased risk for CDI due to more comorbidities, frequent hospitalizations, and age-related immunosuppression.
According to the literature data, clinical cure rate varies from 74 to 97% in CDI patients treated with vancomycin [14, 15]. In the present study, clinical cure was achieved in 79.4% of vancomycin-treated patients, which is in accordance with the results of other authors reporting clinical cure rates of 70%, 79% and 89% in severe forms of the disease [14,15,16].
According to the present study, patients with severe CDI treated with teicoplanin were associated with significantly higher overall clinical cure rate than those treated with vancomycin. Previous studies also demonstrated higher clinical cure and lower recurrence rates in patients treated with teicoplanin compared to those treated with vancomycin [6, 7, 17]. De Lalla et al. showed the efficacy of teicoplanin in patients with pseudomembranous colitis with clinical response of 96.2% and 100% [7, 17]. In the study of Wenish et al., clinical cure was achieved in 96% of teicoplanin-treated patients [6]. All of these studies date back from the period of two decades ago, long before the emergence of hypervirulent strain, NAP1/BI/027, thus, the extrapolation of their results to the current period is difficult. To the best of our knowledge, our study is the first to assess the efficacy of teicoplanin in the treatment of severe CDI in the epidemic era, the only which includes patients with complicated disease, and the largest one addressing teicoplanin usage in CDI ever conducted.
Despite the results of aforementioned studies, teicoplanin was never in widespread use for CDI. Infrequent use and four-fold lower minimum inhibitory concentrations (MICs) of teicoplanin compared to those of vancomycin, might explain higher teicoplanin clinical cure rates, bearing in mind that treatment failure might result from higher MICs of vancomycin and metronidazole reported in certain strains [18, 19].
There are few published data concerning the experience in the treatment of complicated CDI. In a study evaluating the addition of vancomycin enema to standard combination therapy, treatment was successful in 58.3% of patients treated with standard therapy and 54% of those to whom vancomycin enema was added [20]. In a retrospective analysis of the use of tigecycline in combination with standard therapy, clinical cure was achieved in 6/7 (85.7%) patients with complicated disease [21]. In a recent report regarding the use of fidaxomycin in critically ill, treatment response was achieved in 34% of patients with complicated CDI [5].
The clinical cure rate of 73.9% among teicoplanin-treated complicated CDI in the present study is among the highest ever reported for complicated disease. However, this must be interpreted with caution because patients with directly life-threatening disease on admission were excluded from the present study.
The mean time to resolution of diarrhea in the present study was 6.1 ± 3.2 days, and did not differ significantly between the two treatment groups. Time to resolution of diarrhea reported in the literature was shorter, with the median from 58 to 96 h [6, 7, 16, 22]. This discordance could be explained by the fact that other studies included patients not only with severe but also with mild to moderate disease.
In the present study, the all-cause mortality rates in patients with severe as well as in those with complicated CDI were lower in the teicoplanin treatment group, but without statistical significance. Mortality rates reported in the literature range from 4.9% to 35.9% [23, 24]. Patel et al. reported all-cause mortality rate of 18.5% and 37.5% in patients with severe and severe complicated CDI, respectively [25]. In a study addressing severe CDI in patients treated in the intensive care unit, death was reported in 36.7% [26]. The exclusion of patients with life-threatening disease on admission could explain lower mortality rates among patients in the present study.
One of the main issues in management of CDI is high recurrence rate in patients who achieve clinical cure.
Most recurrences occur within the first 30 days after completing a course of CDI therapy [3]. According to Johnson et al., the mean time to relapse was 14.5 days [27]. This is similar to the time to recurrence observed in the present study, which did not differ significantly between treatment groups.
In the present study, the recurrence rate in vancomycin-treated patients was 30.4% and 35% in patients with complicated and non-complicated CDI, respectively. These rates are among higher recurrence rates reported in the literature. According to literature data, in patients treated with vancomycin, recurrence rates can be as high as 38%, while in patients who experienced prior CDI episode these rates even reach 40–65% [22, 28]. These are overall recurrence rates in patients with CDI irrespective of disease severity. The fact that our study included only patients with severe and severe-complicated CDI might be one of the explanations for high recurrence rates in patients treated with vancomycin. The incidence of recurrent CDI reported in the surgical intensive care unit by Jasiak et al. was 43.8% [29]. In a subgroup of patients with severe CDI treated in Italy, five of 17 (29.4%) patients experienced recurrence [30].
The significant percentage of patients with first recurrence in the present study who were included in both treatment groups (63.9% in the vancomycin and 66.4% in the teicoplanin group) is another reason for high recurrence rates in the vancomycin treatment group which approached the recurrence rates reported in the literature for patients with previous CDI. In a study comparing the efficacy of vancomycin and fidaxomycin in the treatment of first recurrence of CDI, recurrence occurred in 35.5% of patients treated with vancomycin [4]. The same category was also analyzed by Pepin et al. and revealed recurrence in 33% of patients [31]. In addition, other authors also reported high recurrence rates after treatment with vancomycin. In a Canadian study recurrence rate was 28% among vancomycin-treated patients [32]. Young et al., Cornely et al. and Louie et al. reported recurrence rates of 33%, 27% and 25%, respectively [16, 22, 33]. In a subgroup of patients with hematological malignancies 41% of patients experienced recurrent CDI [34].
In contrast to the achieved vancomycin recurrence rate, the rate of recurrence in patients treated with teicoplanin was only 9.3%, which was significantly lower in comparison to the vancomycin treatment group. This difference remained significant even after adjustment for confounders, so the use of teicoplanin has proven to be an independent predictor of lower recurrence rate in patients with severe CDI. The previously reported risk factors for recurrence such as advanced age, concomitant antibiotic therapy and comorbidity, were not associated with higher risk for recurrence in our study.
Patients treated with teicoplanin were also less likely to experience recurrence compared to vancomycin recipients in subgroups of patients with complicated CDI, patients who received concomitant antibiotic therapy and those over 65 years old. This is important, because these groups represent patients at highest risk for recurrent disease [3].
The achieved teicoplanin recurrence rates are not only lower than those in the vancomycin treatment group in the present study, but are also remarkably lower than the majority of vancomycin recurrence rates reported in recent studies. They are similar to the results of previous studies of teicoplanin usage in CDI, in which recurrence was observed in up to 7.7% [6, 7, 17]. The results of those studies must be assessed with caution due to the small number of enrolled patients, and cannot be quite comparable with the present study because they were obtained in the pre-epidemic era, before the emergence of hypervirulent strain. The observation that oral teicoplanin might be more effective than vancomycin not only for clinical, but also for bacteriological cure, might explain lower teicoplanin recurrence rates [7].
Recent studies demonstrated that fidaxomycin was associated with lower risk of recurrence in patients with CDI, but this advantage of fidaxomycin was consistently shown only for non-027 ribotypes [16, 22]. The teicoplanin recurrence rate in the present study is comparable to the ones reported in studies with fidaxomycin. Considering the fact that fidaxomycin is not always and everywhere available, teicoplanin might be a potential treatment option in these circumstances, especially in patients with high risk for recurrence.
The presented study has potential limitations. Patients with most severe, directly life-threatening disease were not included in the analysis, because none of them was treated with teicoplanin as initial therapy. In addition, the number of patients with severe complicated disease included in the study was small, but still was among the largest addressing this category of patients reported in a single study. Likewise, this was a non-randomized study, which might have led to selection bias, but the fact that treatment groups did not significantly differ in baseline characteristics contributes to the validity of our results.
In the presented study, molecular characterization of C. difficile strains has not been included, but recently published data showed the remarkable predominance of ribotype 027 in patients in Serbia, so it is most likely that this ribotype was also predominant in the present study population [35]. Therefore, we can speculate that teicoplanin might also be efficient in the treatment of CDI caused by ribotype 027, which was associated with more severe disease and higher mortality and recurrence rates in some previous studies [36]. This additionally emphasizes the potential role of teicoplanin in the treatment of CDI.
According to all the aforementioned, teicoplanin might be a good treatment option for patients with severe CDI. Patients treated with teicoplanin experienced remarkably lower recurrence rates compared to vancomycin-treated patients. In addition, the association of teicoplanin treatment and lower recurrence rates was also demonstrated in patients with advanced age, concomitant antibiotic therapy and those with complicated disease. Because of its higher cost in comparison to vancomycin, teicoplanin might be reserved for patients with the highest risk for recurrence, and those in whom all potential consequences and complications of recurrence could lead to unfavorable and fatal outcome.
Considering the fact that recurrent CDI is a challenge to treat with limited therapeutic options, more studies addressing the use of teicoplanin in the treatment of CDI might be useful. Furthermore, future studies preferably randomized clinical trials, and studies that would include the critically ill and patients with multiple recurrences are required to evaluate the precise role of teicoplanin in the treatment of CDI.
References
De Pestel DD, Aronoff DM (2013) Epidemiology of Clostridium difficile infection. J Pharm Pract 26(5):464–475
Bauer MP, Notermans DW, van Benthem BH et al (2011) Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377:63–73
Kelly CP (2012) Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect 18(Suppl 6):21–27
Cornely OA, Miller MA, Louie TJ et al (2012) Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 55(Suppl 2):S154–S161
Penziner S, Dubrovskaya Y, Press R et al (2015) Fidaxomicin therapy in critically ill patients with Clostridium difficile infection. Antimicrob Agents Chemother 59(3):1776–1781
Wenisch C, Parschalk B, Hasenhündl M et al (1996) Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhoea. Clin Infect Dis 22:813–818
De Lalla F, Nicolin R, Rinaldi E et al (1992) A prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile-associated diarrhoea. Antimicrob Agents Chemother 36:2192–2196
Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases Collaborators (13) (2014) European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 20(Suppl 2):1–26
Popovic N, Korac M, Nesic Z et al (2015) Oral teicoplanin for successful treatment of severe refractory Clostridium difficile infection. J Infect Dev Ctries 9(10):1062–1067
EMA Europa (2017) Targocid- summary of product characteristics, labelling and package leaflet. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Targocid_30/WC500143825.pdf. Accessed 26 December 2017
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Chronic Dis 40(5):373–383
No authors listed (1997) Declaration of Helsinki recommendation guiding physicians in biomedical research involving human subjects. JAMA 277:925–926
Lucado J, Gould C, Elixhauser A (2009) Clostridium difficile infections (CDI) in hospital stays. Healthcare cost and utilization project, Agency for Healthcare Research and Quality. Statistical brief #124. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf. Accessed 26 December 2017
Musher DM, Logan N, Bressler AM et al (2009) Nitazoxanid versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis 48:e41–e46
Zar FA, Bakkanagari SR, Moorthi KMLST et al (2007) A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarhhoea, stratified by disease severity. Clin Infect Dis 45:302–307
Louie TJ, Miller MA, Mullane KM et al (2011) Fidaxomicin versus vancomycin for Clostridium Difficile infection. N Engl J Med 364:422–431
De Lalla, Privitera G, Rinaldi E et al (1989) Treatment of Clostridium difficile-associated disease with teicoplanin. Antimicrob Agents Chemother 33(7):1125–1127
Pantosti A, Luzzi I, Cardines R et al (1985) Comparison of the in vitro activities of teicoplanin and vancomycin against Clostridium difficile and their interactions with cholestyramine. Antimicrob Agents Chemother 28:847–848
Peláez T, Alcalá L, Alonso R et al (2002) Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother 46:1647–1650
Malamood M, Nellis E, Ehrlich AC et al (2015) Vancomycin enemas as adjunctive therapy for Clostridium difficile infection. J Clin Med Res 7(6):422–427
Britt NS, Steed ME, Potter EM et al (2014) Tigecycline for the treatment of severe and severe complicated Clostridium difficile infection. Infect Dis Ther 3(2):321–331
Cornely OA, Crook DW, Esposito R et al (2012) Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 12(4):281–289
Morrison RH, Hall NS, Said M et al (2011) Risk factors associated with complications and mortality in patients with Clostridium difficile infection. Clin Infect Dis 53(12):1173–1178
Wilson V, Cheek L, Satta G et al (2010) Predictors of death after Clostridium difficile infection: a report on 128 strain-typed cases from a teaching hospital in the United Kingdom. Clin Infect Dis 50(12):e77–e81
Patel I, Wungjiranirun M, Theethira T et al (2017) Lack of adherence to SHEA-IDSA treatment guidelines for Clostridium difficile infection is associated with increased mortality. J Antimicrob Chemother 72:574–581
Kenneally C, Rosini JM, Skrupky LP et al (2007) Analysis of 30-day mortality for clostridium difficile-associated disease in the ICU setting. Chest 132(2):418–424
Johnson S, Adelmann A, Clabots CR et al (1989) Recurrence of Clostridium difficile diarrhoea not caused by the original infecting organism. J Infect Dis 159:340–343
Mc Farland LV, Elmer GW, Surawicz CM (2002) Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gatroenterol 97:1769–1775
Jasiak NM, Alaniz C, Rao K, Veltman K, Nagel JL (2016) Recurrent Clostridium difficile infection in intensive care unit patients. Am J Infect Control 44(1):36–40
Di Bella S, Paglia MG, Johnson E, Petrosillo N (2012) Clostridium difficile 027 infection in Central Italy. BMC Infect Dis 12:370
Pepin J, Alary ME, Valiquette L et al (2005) Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis 40:1591–1597
Lee C, Louie TJ, Weiss K, Valiquette L, Gerson M, Arnott W, Gorbach SL (2016) Fidaxomicin versus Vancomycin in the treatment of Clostridium difficile infection: Canadian outcomes. Can J Infect Dis Med Microbiol 118:725–732
Young GP, Ward PB, Bayley N et al (1985) Antibiotic-associated colitis due to Clostridium difficile: double-blind comparison of vancomycin with bacitracin. Gastroenterology 89:1038
Scappaticci GB, Perissinotti AJ, Nagel JL, Bixby DL, Marini BL (2017) Risk factors and impact of Clostridium difficile recurrence on haematology patients. J Antimicrob Chemother 72(5):1488–1495
Rupnik M, Tambic Andrasevic A, Trajkovska Dokic E et al (2016) Distribution of Clostridium difficile PCR ribotypes and high proportion of 027 and 176 in some hospitals in four south eastern European countries. Anaerobe 42:142–144
Muto CA, Pokrywka M, Shutt K et al (2005) A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol 26:273–280
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors declare that they have no conflict of interest. The authors received no financial support for this research.
All procedures performed involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants (or their caregivers) included in the study.
Electronic supplementary material
ESM 1
(SAV 71 kb)
Rights and permissions
About this article
Cite this article
Popovic, N., Korac, M., Nesic, Z. et al. Oral teicoplanin versus oral vancomycin for the treatment of severe Clostridium difficile infection: a prospective observational study. Eur J Clin Microbiol Infect Dis 37, 745–754 (2018). https://doi.org/10.1007/s10096-017-3169-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3169-3