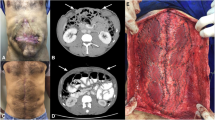
Abstract
Background
Abdominal wall reconstruction in patients presenting with enteric fistulas and mesh infection is challenging. There is a consensus that synthetic mesh must be avoided in infected operations, and the alternatives to using synthetic mesh, such as component separation techniques and biologic mesh, present disappointing results with expressive wound infection and hernia recurrence rates.
Methods
A prospective clinical trial designed to evaluate the short- and long-term outcomes of 40 patients submitted to elective abdominal wall repair with synthetic mesh in the dirty-infected setting, and compared to a cohort of 40 patients submitted to clean ventral hernia repairs. Patients in both groups were submitted to a single-staged repair using onlay polypropylene mesh reinforcement.
Results
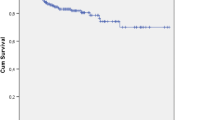
Groups' characteristics were similar. There were 13 (32.5%) surgical site occurrences in the infected mesh (IM) group, compared to 11 (27.5%) in the clean-control (CC) group, p = 0.626. The 30-day surgical site infection rate was 15% for the IM group vs. 10% for the CC cases, p = 0.499. One patient required a complete mesh removal in each group. The mean overall follow-up was 50.2 ± 14.8 months, with 36 patients in the IM group and 38 clean-controls completing a follow-up of 36 months. There was one hernia recurrence (4.2%) in the IM group and no recurrences in the CC group.
Conclusion
We demonstrated that using polypropylene mesh in the infected setting presented similar outcomes to clean repairs. The use of synthetic mesh in the onlay position resulted in a safe and durable abdominal wall reconstruction.
Trial registration
Study registered at Plataforma Brasil (plataformabrasil.saude.gov.br), CAAE 30836614.7.0000.0068. Study registered at Clinical Trials (clinicaltrials.gov), Identifier NCT03702153.
Similar content being viewed by others
References
The Ventral Hernia Working Group, Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D (2010) Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 148:544–558
Itani KM, Rosen MJ, Vargo D, Awad SS, DeNoto G III, Butler CE (2012) Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery 152:498–505
Rosen MJ, Bauer JJ, Harmaty M, Carbonell AM, Cobb WS, Matthews B, Goldblat MI, Selzer DJ, Poulose BK, Hansson BM, Rosman C, Chao JJ, Jacobsen GR (2017) Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: The COBRA Study. Ann Surg 265:205–211
Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ (2013) Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg 217:991–998
Atema JJ, de Vries FEE, Boermeester MA (2016) Systematic review and meta-analysis of the repair of potentially contaminated and contaminated abdominal wall defects. Am J Surg 212:982–995
Voyles CR, Richardson JD, Bland KI, Tobin GR, Flint LM, Polk HC Jr (1981) Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg 194:219–223
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Basoglu M, Yildirgan MI, Yilmaz I, Balik A, Celebi F, Atamanalp SS, Polak KY, Oren D (2004) Late complications of incisional hernias following prosthetic mesh repair. Acta Chir Belg 104:425–428
Sahoo S, Haskins IN, Huang LC, Krpata DM, Derwin KA, Poulouse BK, Rosen MJ (2017) Early wound morbidity after open ventral hernia repair with biosynthetic or polypropylene mesh. J Am Coll Surg 225:472–480
Garner JS (1986) CDC guidelines for prevention of surgical wound infections, 1985. Supersedes guidelines for prevention of surgical wound infections published in 1982. Infect Control 7:193–200 (originally published in November 1985)
Muysoms F, Campanelli G, Champault GG, DeBeaux AC, Dietz UA, Jeekel J, Klinge U, Köckerling F, Mandala V, Montgomery A, Morales Conde S, Puppe F, Simmermacher RK, Śmietański M, Miserez M (2012) EuraHS: the development of an international online platform for registration and outcome measurement of ventral abdominal wall hernia repair. Hernia 16:239–250
Tanaka EY, Yoo JH, Rodrigues AJ Jr, Utiyama EM, Birolini D, Rasslan S (2009) A computerized tomography scan method for calculating the hernia sac and abdominal cavity volume in complex large incisional hernia with loss of domain. Hernia 14:63–69
Haskins IN, Horne CM, Krpata DM, Prabhu AS, Tastaldi L, Perez AJ, Rosenblatt S, Poulose BK, Rosen MJ (2018) A call for standardization of wound events reporting following ventral hernia repair. Hernia 22:729–736
STATACorp (2007) Stata Statistical Software: Release 10.0. Stata Corporation, College Station
Rosen MJ, Krpata DM, Ermlich B, Blatnik JA (2013) A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 257:991–996
Birolini C, Utiyama EM, Rodrigues AJ Jr, Birolini D (2000) Elective colonic operation and prosthetic repair of incisional hernia: does contamination contraindicate abdominal wall prosthesis use? J Am Coll Surg 191:366–372
Kelly ME, Behrman SW (2002) The safety and efficacy of prosthetic hernia repair in clean-contaminated and contaminated wounds. Am Surg 6:524–529
Geisler DJ, Reilly JC, Vaughan SG, Glennon EJ, Kondylis PD (2003) Safety and outcome of use of nonabsorbable mesh for repair of fascial defects in the presence of open bowel. Dis Colon Rectum 46:1118–1123
Campanelli G, Nicolosi FM, Pettinari D, Contessini Avesani E (2004) Prosthetic repair, intestinal resection, and potentially contaminated areas: safe and feasible? Hernia 8:190–192
Majumder A, Winder JS, Wen Y, Pauli EM, Belyansky I, Novitsky YW (2016) Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery 160:828–838
Franklin ME Jr, Trevino JM, Portillo G, Vela I, Glass JL, Gonzalez JJ (2008) The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow-up. Surg Endosc 22:1941–1946
Machairas A, Liakakos T, Patapis P, Petropoulos C, Tsapralis D, Misiakos EP (2008) Prosthetic repair of incisional hernia combined with elective bowel operation. Surgeon 6:274–277
Garvey PB, Martinez RA, Baumann DP, Liu J, Butler CE (2014) Outcomes of abdominal wall reconstruction with acellular dermal matrix are not affected by wound contamination. J Am Coll Surg 219:853–864
Madani A, Niculiseanu P, Marini W, Kaneva PA, Mappin-Kasirer B, Vassiliou MC, Khwaja K, Fata P, Fried GM, Feldman LS (2017) Biologic mesh for repair of ventral hernias in contaminated fields: long-term clinical and patient-reported outcomes. Surg Endosc 31:861–871
Huntington CR, Cox TC, Blair LJ, Schell S, Randolph D, Prasad T, Lincourt A, Heniford BT, Augenstein VA (2016) Biologic mesh in ventral hernia repair: outcomes, recurrence and charge analysis. Surgery 160:1517–1527
Atema JJ, Furnee EJ, Maeda Y, Warusavitarne J, Tanis PJ, Bemelman WA, Valzey CJ, Boermeester MA (2017) Major complex abdominal wall repair in contaminated fields with use of a non-cross-linked biologic mesh: a dual-institutional experience. World J Surg 41:1993–1999
Birolini C, de Miranda JS, Utiyama EM, Rasslan S (2015) A retrospective review and observations over a 16-year clinical experience on the surgical treatment of chronic mesh infection. What about replacing a synthetic mesh on the infected surgical field? Hernia 19:239–246
Connoly PT, Teubner A, Lees NP, Anderson ID, Scott NA, Carlson GL (2008) Outcome of reconstructive surgery for intestinal fistula in the open abdomen. Ann Surg 247:440–444
Slater NJ, Bokkerink WJV, Konijn V, Bleichrodt RP, van Goor H (2015) Safety and durability of one-stage repair of abdominal wall defects with enteric fistulas. Ann Surg 261:553–557
Wind J, van Koperen PJ, Slors JF, Bemelman WA (2009) Single-stage closure of enterocutaneous fistula and stomas in the presence of large abdominal wall defects using the components separation technique. Am J Surg 197:24–29
Kugler NW, Bobbs M, Webb T, Carver TW, Milia D, Paul JS (2016) A dual-stage approach to contaminated, high-risk ventral hernia repairs. J Surg Res 204:200–204
van Geffen HJAA, Simmermacher RKJ, van Vroonhoven TJMV, van der Werken C (2005) Surgical treatment of large contaminated abdominal wall defects. J Am Col Surg 201:206–212
Krpata DM, Stein SL, Eston M, Ermlich B, Blatnik JA, Novitsky YM, Rosen MJ (2013) Outcomes of simultaneous large complex abdominal wall reconstruction and enterocutaneous fistula take-down. Am J Surg 205:354–359
Zerbib P, Caiazzo R, Piessen G, Rogosnotzky M, Sequier C, Koriche D, Truant S, Boleslawski E, Chambom JP, Pruvot FR (2015) Outcome in porcine acellular dermal matrix reinforcement of infected abdominal wall defects: a prospective study. Hernia 19:253–257
Pandey H, Thakur DS, Somashekar U, Kothary R, Agarwal P, Sharma D (2018) Use of polypropylene mesh in contaminated and dirty strangulated hernias: short term results. Hernia 22:1045–1050
Birolini C, de Miranda JS, Utiyama EM, Rasslan S, Birolini D (2016) Active Staphylococcus aureus infection: Is it a contra-indication to the repair of complex hernias with synthetic mesh? A prospective observational study on the outcomes of synthetic mesh replacement, in patients with chronic mesh infection caused by Staphylococcus aureus. Int J Surg 28:56–62
Arnold MR, Kao AM, Gbozah KK, Heniford BT, Augenstein VA (2018) Optimal management of mesh infection: evidence and treatment options. Int J Abdom Wall Hernia Surg 1:42–49
Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YM, Rosen MJ (2012) In vivo analysis of the morphological characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg 16:2139–2144
Funding
No external funds or financial support were used for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has conflicts of interests to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Birolini, C., de Miranda, J.S., Tanaka, E.Y. et al. The use of synthetic mesh in contaminated and infected abdominal wall repairs: challenging the dogma—A long-term prospective clinical trial. Hernia 24, 307–323 (2020). https://doi.org/10.1007/s10029-019-02035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-019-02035-2