Abstract
Background: Supplementary polyglecaprone 25 (Monocryl) monofilaments were added to a lightweight pure monofilament polypropylene mesh (PP mesh) to improve intraoperative handling (PP+M mesh). This study was designed to evaluate the influence of this additional supplementation on the biocompatibility in a rodent animal model.
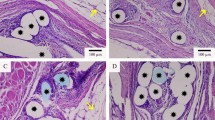
Methods: Two mesh materials, a composite mesh (PP+M) and the pure polypropylene variant (PP), were compared after subcutaneous implantation in a standardized rat model. Histological analysis of the inflammatory response was performed after 28, 56 and 84 days of implantation. Material absorption, inflammatory tissue reaction, fibrosis and granuloma formation were investigated, as well as the percentage of proliferating and apoptotic cells at the interface.
Results: Both mesh materials showed a slight foreign body reaction involving mainly macrophages and foreign body giant cells. Total absorption of the Monocryl filaments of the PP+M mesh occurred between 56 and 84 days of implantation. Both the inflammatory and the fibrotic reaction were decreased (n.s.) in the PP+M mesh group compared to the pure PP mesh. Whereas the percentage of proliferating cells showed no significant difference, the rate of apoptotic cells was significantly decreased in the PP+M mesh group over the whole implantation period.
Conclusion: Compared to the pure polypropylene mesh, our data confirm that the use of a polypropylene mesh supplemented with absorbable Monocryl filaments is feasible without additional short-term mesh-related complications in the experimental model or negative side effects on biocompatibility.





Similar content being viewed by others
References
Rios A, Rodriguez JM, Munitiz V, Alcaraz P, Perez FD, Parrilla P (2001) Antibiotic prophylaxis in incisional hernia repair using a prosthesis. Hernia 5:148–152
Klinge U, Klosterhalfen B, Conze J, Limberg W, Obolenski B, Ottinger AP, Schumpelick V (1998) Modified mesh for hernia repair that is adapted to the physiology of the abdominal wall. Eur J Surg 164:951–960
Schumpelick V, Klosterhalfen B, Muller M, Klinge U (1999) Minimized polypropylene mesh for preperitoneal net plasty (PNP) of incisional hernias. Chirurg 70:422–430
Bringman S, Heikkinen TJ, Wollert S, Osterberg J, Smedberg S, Granlund H, Ramel S, Fellander G, Anderberg B (2004) Early results of a single-blinded, randomized, controlled, Internet-based multicenter trial comparing Prolene and Vypro II mesh in Lichtenstein hernioplasty. Hernia 8:127–134
Post S, Weiss B, Willer M, Neufang T, Lorenz D (2004) Randomized clinical trial of lightweight composite mesh for Lichtenstein inguinal hernia repair. Brit J Surg 91:44–48
Klinge U, Klosterhalfen B, Muller M, Anurov M, Ottinger A, Schumpelick V (1999) Influence of polyglactin-coating on functional and morphological parameters of polypropylene-mesh modifications for abdominal wall repair. Biomaterials 20:613–623
Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V (2002) Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res 63:765–771
Junge K, Klinge U, Rosch R, Klosterhalfen B, Schumpelick V (2002) Functional and morphologic properties of a modified mesh for inguinal hernia repair. World J Surg 26:1471–1480
Bezwada RS, Jamiolkowski DD, Lee IY, Agarwal V, Persivale J, Trenka-Benthin S, Erneta M, Suryadevara J, Yang A, Liu S (1995) Monocryl suture, a new ultra-pliable absorbable monofilament suture. Biomaterials 16:1141–1148
LaBagnara J Jr (1995) A review of absorbable suture materials in head & neck surgery and introduction of monocryl: a new absorbable suture. Ear Nose Throat J 74:409–415
Kirpensteijn J, Maarschalkerweerd RJ, Koeman JP, Kooistra HS, van Sluijs FJ (1997) Comparison of two suture materials for intradermal skin closure in dogs. Vet Quart 19:20–22
Niessen FB, Spauwen PH, Kon M (1997) The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-rapide. Ann Plas Surg 39:254–260
Nary FH, Matsumoto MA, Batista AC, Lopes LC, de Goes FC, Consolaro A (2002) Comparative study of tissue response to polyglecaprone 25, polyglactin 910 and polytetrafluorethylene suture materials in rats. Braz Dent J 13:86–91
Scheidbach H, Tamme C, Tannapfel A, Lippert H, Kockerling F (2004) In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: an experimental study in pigs. Surg Endosc 18:211–220
Coda A, Bendavid R, Botto-Micca F, Bossotti M, Bona A (2003) Structural alterations of prosthetic meshes in humans. Hernia 7:29–34
Rosch R, Junge K, Quester R, Klinge U, Klosterhalfen B, Schumpelick V (2003) Vypro II mesh in hernia repair: impact of polyglactin on long-term incorporation in rats. Eur Surg Res 35:445–450
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG Kl 1320/2–1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junge, K., Rosch, R., Krones, C.J. et al. Influence of polyglecaprone 25 (Monocryl) supplementation on the biocompatibility of a polypropylene mesh for hernia repair. Hernia 9, 212–217 (2005). https://doi.org/10.1007/s10029-004-0315-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-004-0315-5