Abstract
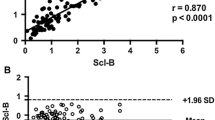
Sclerostin is a Wnt inhibitor produced specifically by osteocytes. It decreases bone formation by repressing osteoblast differentiation and proliferation. Whether circulating sclerostin level is affected by liver function is not currently clear. The aim of the study was to evaluate this relationship. Our cross-sectional study included 47 patients with liver cirrhosis and 50 healthy controls. Serum sclerostin level was analyzed by ELISA. Serum sclerostin levels were significantly higher in patients with cirrhosis than in controls (50.8 ± 38.2 vs. 35.1 ± 8.8 pmol/L, p = 0.008). After further adjustment for age, sex, body mass index, serum creatinine, and presence of diabetes, cirrhosis patients had higher sclerostin than controls. Subgroup analysis found that patients with Child–Pugh class B or C had higher sclerostin levels than patients with class A or controls after adjusting for multiple confounding factors. Multiple regression analysis showed that gender (p = 0.022), presence of diabetes (p < 0.001), albumin (p = 0.010), and serum creatinine (p = 0.037) were independent factors for circulating sclerostin level. Circulating sclerostin was higher in patients with advanced liver cirrhosis than in healthy controls or patients with early liver cirrhosis. The elevated sclerostin levels clearly correlated with markers of liver dysfunction such as albumin. The relationship between circulating sclerostin and liver function indicates a possible role of the liver in sclerostin metabolism.

Similar content being viewed by others
References
Collier J (2007) Bone disorders in chronic liver disease. Hepatology 46:1271–1278
Leslie WD, Bernstein CN, Leboff MS, American Gastroenterological Association Clinical Practice Committee (2003) AGA technical review on osteoporosis in hepatic disorders. Gastroenterology 125:941–966
Nakchbandi IA, van der Merwe SW (2009) Current understanding of osteoporosis associated with liver disease. Nat Rev Gastroenterol Hepatol 6:660–670
Foresta C, Schipilliti M, Ciarleglio FA, Lenzi A, D’Amico D (2008) Male hypogonadism in cirrhosis and after liver transplantation. J Endocrinol Invest 32:470–482
Guañabens N, Parés A, Mariñoso L, Brancós MA, Piera C, Serrano S, Rivera F, Rodés J (1990) Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol 85:1356–1362
Stellon AJ, Webb A, Compston J, Williams R (1987) Low bone turnover state in primary biliary cirrhosis. Hepatology 7:137–142
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J (2005) Sclerostin is a delayed secreted products of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Baron R, Rawadi G (2007) Targeting the Wnt/β-catenin pathway to regulate bone formation in the adults skeleton. Endocrinology 148:2635–2643
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887
Mödder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ 3rd, Khosla S (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379
Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR, Fahrleitner-Pammer A (2012) Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab 97:148–154
Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE (2010) Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95:2248–2253
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the esophagus for bleeding esophageal varices. Br J Surg 60:646–649
González-Reimers E, Martín-González C, de la Vega-Prieto MJ, Pelazas-González R, Fernández-Rodríguez C, López-Prieto J, Alvisa-Negrín J, Santolaria-Fernández F (2013) Serum sclerostin in alcoholics: a pilot study. Alcohol Alcohol 48:278–282
Mirza FS, Padhi ID, Raisz LG, Lorenzo JA (2010) Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab 95:1991–1997
Mödder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S (2011) Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res 26:27–34
Lapauw B, Vandewalle S, Taes Y, Goemaere S, Zmierczak H, Collette J, Kaufman JM (2013) Serum sclerostin levels in men with idiopathic osteoporosis. Eur J Endocrinol 168:615–620
García-Fontana B, Morales-Santana S, Varsavsky M, García-Martín A, García-Salcedo JA, Reyes-García R, Muñoz-Torres M (2013) Sclerostin serum levels in prostate cancer patients and their relationship with sex steroids. Osteoporos Int [Epub ahead of print] doi 10.1007/s00198-013-2462-y
Bannister P, Losowky MS (1988) Sex hormones and chronic liver disease. J Hepatol 6:258–262
Kaymakoğlu S, Okten A, Cakaloğlu Y, Boztaş G, Beşişik F, Taşçioğlu C, Yalçin S (1995) Hypogonadism is not related to the etiology of liver cirrhosis. J Gastroenterol 30:745–750
Galvão-Teles A, Burke CW, Anderson DC, Marshall JC, Corker CS, Bown RL, Clark ML (1973) Biologically active androgens and oestradiol in men with chronic liver disease. Lancet 1:173–177
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH (2008) Mechanical stimulation of bone in vivo reduces osteocytes expression of Sost/sclerostin. J Biol Chem 293:5866–5875
Diamond TH, Stiel D, Lunzer M, McDowall D, Eckstein RP, Posen S (1989) Hepatic osteodystrophy. Static and dynamic bone histomorphometry and serum bone Gla-protein in 80 patients with chronic liver disease. Gastroenterology 96:213–221
Cemborain A, Castilla-Cortázar I, García M, Muguerza B, Delgado G, Díaz-Sánchez M, Picardi A (2000) Effects of IGF-I treatment on osteopenia in rats with advanced liver cirrhosis. J Physiol Biochem 56:91–99
Kawelke N, Bentmann A, Hackl N, Hager HD, Feick P, Geursen A, Singer MV, Nakchbandi IA (2008) Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J Bone Miner Res 23:1278–1286
Xu G, Niki T, Virtanen I, Rogiers V, De Bleser P, Geerts A (1997) Gene expression and synthesis of fibronectin isoforms in rat hepatic stellate cells. Comparison with liver parenchymal cells and skin fibroblasts. J Pathol 183:90–98
García-Martín A, Rozas-Moreno P, Reyes-García R, Morales-Santana S, García-Fontana B, García-Salcedo JA, Muñoz-Torres M (2012) Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 97:234–241
Gaudio A, Privitera F, Battaglia K, Torrisi V, Sidoti MH, Pulvirenti I, Canzonieri E, Tringali G, Fiore CE (2012) Sclerostin levels associated with inhibition of the Wnt/β-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab 97:3744–3750
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rhee, Y., Kim, W.J., Han, K.J. et al. Effect of liver dysfunction on circulating sclerostin. J Bone Miner Metab 32, 545–549 (2014). https://doi.org/10.1007/s00774-013-0524-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0524-z