Abstract
Background
Mesh augmentation is a highly controversial adjunct of hiatus hernia (HH) surgery. The current scientific evidence remains unclear and even experts disagree on indications and surgical techniques. With an aim to avoid the downsides of both non-resorbable synthetic and biological materials, biosynthetic long-term resorbable meshes (BSM) have recently been developed and are becoming increasingly popular. In this context, we aimed at assessing outcomes after HH repair with this new generation of mesh at our institution.
Methods
From a prospective database, we identified all consecutive patients that underwent HH repair with BSM augmentation. Data was extracted from electronic patient charts of our hospital information system. Endpoints of this analysis included perioperative morbidity, functional results and recurrence rates at follow-up.
Results
Between December 2017 and July 2022, 97 patients (elective primary cases n = 76, redo cases n = 13, emergency cases n = 8) underwent HH with BSM augmentation. Indications in elective and emergency cases were paraesophageal (Type II–IV) HH in 83%, and large Type I HH in 4%. There was no perioperative mortality, and overall (Clavien–Dindo ≥ 2) and severe (Clavien–Dindo ≥ 3b) postoperative morbidity was 15% and 3%, respectively. An outcome without postoperative complications was achieved in 85% of cases (elective primary surgery 88%, redo cases 100%, emergencies cases 25%). After a median (IQR) postoperative follow-up of 12 months, 69 patients (74%) were asymptomatic, 15 (16%) reported improvement, and 9 (10%) had clinical failure, of which 2 patients (2%) required revisional surgery.
Conclusion
Our data suggest that HH repair with BSM augmentation is feasible and safe with low perioperative morbidity and acceptable postoperative failure rates at early to mid-term follow-up. BSM may be a useful alternative to non-resorbable materials in HH surgery.
Similar content being viewed by others
The problem of herniation after sutured closure of human fascia is as old as modern surgery. In the 1870s, it was Theodor Billroth who stated: “If we could artificially produce tissue of the density and toughness of fascia and tendon, the secret of radical hernia cure would be discovered”. In 1957, the discovery to synthesize polypropylene at an industrial scale [1] was the stepping stone for the success of surgical mesh reinforcement, which has become the uncontested standard of care for incisional and inguinal hernia, rectal and vaginal prolapse, and many other hernia types [2,3,4,5,6].
However, mesh reinforcement in hiatus hernia (HH) surgery has always been controversial, and many surgeons avoid crural prostheses because of the lack of convincing scientific evidence, the risk of severe complications, and the technical challenges of revisional surgery [7]. On the other hand, mesh supporters argue that augmentation should be performed particularly in large HH to reduce the high risk of long-term recurrence and, most importantly, to avoid complex reoperations [8]. In addition, mesh-related morbidity such as stenosis and erosion appears to be rare with a reported incidence of 0.035% [9], and severe complications occur almost exclusively after reinforcement with non-absorbable synthetic materials [10].
In an attempt to overcome the undesirable characteristics of permanent synthetic meshes, absorbable allogenic and xenogeneic materials (“biomeshes”) have been introduced and widely promoted. Biomesh is rapidly revascularized and has a high resistance to bacterial contamination [11, 12]. However, high cost, negative long-term results in randomized trials [13], and cultural and religious issues [14] have prevented widespread use of these materials.
New-generation long-term absorbable biosynthetic meshes (BSM) have recently been developed to combine the advantages and avoid the downsides of synthetic materials and biomeshes. Phasix ST® (BD, Allschwil, Switzerland) is made from poly-4-hydroxybutyrate (P4HB), a material that handles well laparoscopically, absorbs and remodels to native host tissue within 6–18 months [15, 16], and should therefore carry a lower risk of long-term complications. Although promising in concept, only few studies have reported clinical outcomes after P4HB reinforcement in HH repair [17,18,19]. Hence, the aim of this study was to analyze our institutional experience with this new generation of mesh.
Methods
Study design
From a prospective database, we identified all consecutive patients that underwent HH repair with BSM augmentation at our institution. Data relevant to this study such as basic patient demographics, pre- and postoperative clinical work-up, and details of surgical therapy were gathered from both our prospective database and the electronic patient charts of our hospital information system. The study was approved by the Ethical Committee of the Canton of Zurich (BASEC Nr.: 2021-00822).
Preoperative work-up and surgical technique
Routine preoperative work-up included endoscopy and radiography (contrast swallow or CT-scan). In addition, functional investigations (esophageal pH monitoring and manometry) were performed in patients with esophagitis, reflux symptoms, and dysphagia. In contrast, esophageal pH monitoring and manometry were facultative in large Type III or Type IV HH, particularly in cases with chronic anemia and Cameron lesions.
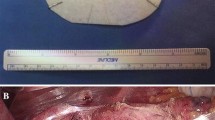
All surgical procedures were performed in a modified “French position” with 20–25° anti-Trendelenburg tilt and the surgeon standing between the patient’s legs. Five trocars were used for the laparoscopic approach. Dissection of the esophageal hiatus usually started at the right crus after division of the lesser omentum. After identification of the phreno-esophageal membrane and dissection of the hernia sac (if present), mediastinal mobilization was performed until the intraabdominal esophageal segment reached a sufficient length of 2–3 cm without longitudinal traction. Reconstruction of the hiatus was then performed in all patients with a posterior crurorrhaphy using 3–4 single form-8 stitches (Ethibond 0, Ethicon, Zug, Switzerland) and—in large hiatus defects—an additional left anterior cruroplasty with the same suture material. One patient underwent an additional Collis procedure for esophageal lengthening. No diaphragmatic relaxing incisions were performed. For mesh augmentation, a circular (8 cm diameter) monofilament P4HB patch with a hydrogel barrier on the abdominal surface (Phasix™ ST Mesh, BD) was used. Prior to implantation, the patch was modified with a 2.5–3 cm central recess for the esophagus via a radial incision. The central recess was placed 1–2 cm caudally from the center of the patch to create a wider cover on the anterior hiatal circumference, which was considered the weakest point of the reconstruction. The radial incision to create the recess was placed posteriorly to provide uninterrupted anterior cover of the hiatus (Fig. 1a). After placement around the abdominal esophagus in an onlay fashion, the mesh was fixed at the hiatus with 4–6 absorbable sutures (Vicryl 3-0, Ethicon). Additional sphincter augmentation with total or partial fundoplication, magnetic sphincter augmentation, fundo-phrenicopexy, His-angle reconstruction (Lortat-Jacob), or Hill gastropexy was performed according to the patients’ individual pathophysiology, symptom profile, and surgeons’ preference.
a Intraoperative aspect of the esophageal hiatus reinforced with circular Phasix ST™ (BD) patch. b Aspect of the circular Phasix ST™ (BD) patch. Note that the central recess is placed 1-2 cm below the center of the patch to achieve a wider covering of the anterior hiatus. Markings to guide mesh positioning in German language L Leber (liver) and M Milz (spleen)
Postoperative follow-up and outcome measures
All patients were offered routine functional aftercare including standardized assessment of symptoms and contrast UGI radiography. In addition, patients with esophagitis or Barrett’s esophagus were routinely followed up endoscopically. Outpatient visits were scheduled at 6 weeks, 3 months and then annually. All patients who missed routine follow-up visits were contacted and invited for an individual appointment at our outpatient clinic. Outcome measures included postoperative morbidity, intensive care unit (ICU) and hospital stay, hospital readmission, and failure rates (clinical, radiological, and endoscopic). Postoperative complications were graded according to the Clavien–Dindo (CD) classification [20] and the Comprehensive Complication Index (CCI) at 30 and 90 days after surgery [21]. Recurrence was defined as any size hernia identified on postoperative UGI radiography or endoscopy.
Statistical analysis
Numerical variables were summarized using medians and interquartile ranges (IQR). Categorical variables were summarized using counts and percentages. The t- or Wilcoxon tests (continuous variables) or chi-square/Fisher tests (proportions) were used as appropriate. Linear mixed-effects models, which account for correlated (within patient) data, was used to evaluate changes in patients’ QoL over time. Overall recurrence rates were projected using Kaplan–Meier estimation and 95% confidence intervals. For all testing, a p < 0.05 was considered statistically significant. All statistical analysis was performed with IBSM SPSS software (version 24.0, SPSS Inc, Armonk, NY, USA).
Results
Patient characteristics and surgical procedures
Between December 2017 and July 2022, 97 patients underwent HH repair with BSM reinforcement at our department. Basic patient demographics, preoperative symptom profiles, and details of the preoperative work-up are summarized in Table 1. The predominant indication for surgery was a primary Type III hiatus hernia (77%). Emergencies and recurrent hernia accounted for 8% and 13% of cases, respectively. Redo cases had a history of one or two previous HH repairs in 11% and 3%, respectively.
Most patients underwent laparoscopic access surgery (92%) in an elective setting (92%). In all cases, a circular Phasix™ ST (BD) mesh was placed around the esophagus in a circular fashion and fixed with absorbable sutures. Details of surgical interventions including all concomitant procedures are displayed in Table 2.
Perioperative outcomes
The median ICU and hospital stay was 0 and 4 days, respectively. Only emergency cases required ICU care. All intra- and postoperative complications are listed in Table 3. An outcome without postoperative complications in elective primary, redo, and emergencies cases was achieved in 88%, 100%, and 25%, respectively. Mortality was 0% within 30 days after surgery, and overall (CD ≥ 2) and severe (CD ≥ 3b) morbidity was 15% and 3%, respectively. Perioperative morbidity was significantly (p < 0.05) higher in emergency cases. No intraoperative complications and no intra- and postoperative mesh-related morbidity was encountered.
Outcomes at follow-up
The median (IQR) follow-up was 12 [17] months, 93 patients (96%) were followed up regularly, and 4 patients (4%) were lost to follow up. At follow-up, complete success (defined as being asymptomatic without evidence of anatomical HH recurrence) was achieved in 69 patients (74%), partial success (defined as symptom improvement with or without anatomical HH recurrence or asymptomatic anatomical HH recurrence) was achieved in 15 patients (16%), and 9 patients (10%) reported clinical failure (defined as unchanged or worsened symptoms with or without anatomical recurrence). A detailed symptom analysis in patients with clinical failure is outlined in Table 4.
Upper gastrointestinal endoscopy and/or contrast swallow studies were performed in 25 (27%) and 55 patients (59%) at follow-up, respectively. Anatomical HH recurrence was evidenced in 8 patients (9%). Two patients required surgical revision for symptomatic hernia recurrence after 23 and 34 months, respectively. At reoperation, complete absorption of the mesh with mild adhesions to adjacent organs was noted.
Discussion
The present study is currently the largest reporting on BSM augmentation in HH repair and adds further evidence to the existing literature. Similar to earlier series using P4HB patches [17,18,19], we observed no severe mesh-related intra- and perioperative morbidity, and clinical results after mid- and long-term follow-up were encouraging with good functional results, a low objective recurrence rate and no evidence of erosions or stenosis. In agreement with the previously published experience, we may therefore conclude that BSM with P4HB patches can be safely performed at the hiatus. [2,3,4,5,6,7, 9, 22].
Although—from a physical point of view—it may seem obvious that meshes increase the tensile strength of the reconstructed hiatus, the general indication for mesh augmentation in HH surgery remains a hotly contested topic. In this regard, the current evidence from randomized controlled trials (RCT) [8, 13, 23,24,25,26,27,28] and meta-analyses [29,30,31,32,33] shows no clear advantage of mesh augmentation compared with sutured closure alone during short- and long-term follow-up. Nevertheless, the available evidence is difficult to interpret owing to different indications, mesh materials, surgical techniques, definitions of recurrence, and durations of follow-up. Indeed, mesh augmentation at the hiatus is far from standardized and there are various materials (synthetic, biosynthetic, biological, absorbable, and non-absorbable) with diverging characteristics on the market. Meshes come in many different sizes and shapes (rectangular, circular, u-shaped) and can be placed at the hiatus in keyhole, posterior, or anterior fashion or on separate (relaxing) diaphragmatic incisions. Therefore, we may assume that the conflicting evidence regarding mesh augmentation at the hiatus in general is at least in part caused by the technical heterogeneity of the surgical approaches [29, 34].
In this context, it may be revealing to review the relationship between anatomical patterns of HH recurrence and the duration of follow-up. From several well-performed retrospective cohort studies we know that most early recurrences (< 12 months) [35, 36], are typically located posteriorly or circumferentially in consequence of a disrupted crurorrhaphy and may therefore be considered a technical failure; i.e. a true recurrence [37]. In contrast, recurrent HH after long-term follow-up (> 12 months) seems to have a different pathophysiology as it is mostly found at the anterior hiatus [37,38,39] as a result of the constant physiological strain leading to stretch and widening of the weakest part of the hiatus over time. We agree with others [40] that early and late HH recurrence should therefore be seen as separate entities, and we would like to stress that this distinction must be considered when analyzing the current literature.
In this context, there is growing evidence from the literature that circular mesh placement leads to fewer recurrences compared with u-shaped posterior mesh configuration [41]. On the other hand, “keyhole” placement of non-absorbable materials is avoided by many surgeons because of the risk of mesh shrinkage and stenosis at follow-up. Therefore, mesh augmentation has been performed posterior-only in most published RCTs without adequate reinforcement of the anterior “weak spot” of the hiatus. As a result, long-term HH recurrence rates were similar or even higher after mesh augmentation in the “posterior-only” RCTs [13, 26,27,28, 42], but significantly lower in RCTs employing circular [23] or combined posterior and anterior mesh reinforcement [25]. We may therefore speculate that insufficient anterior augmentation in most included RCTs may have contributed to the negative results of a recent meta-analysis [29].
With this in mind, and considering the fact that up to 75% of recurrences are located anteriorly, we have changed our institutional technique of mesh configuration by placing the recess for the abdominal esophagus 1–2 cm below the center of the patch to achieve an even wider coverage of the anterior hiatus (Fig. 1b).
There are certain limitations associated with our study. Most importantly, as this is a prospective cohort study of consecutive cases undergoing BSM augmentation, we were not able to compare patients to a “suture only” control group. Thus, an adequately powered registry analysis or preferably an RCT with clearly defined and standardized indications, surgical procedures and quality assurance would be the next step in evaluating the therapeutic strategies for HH repair.
In conclusion, this study confirms that reinforcement of crurorrhaphy with the Phasix ST® (BD) P4HB patch is feasible and clinically effective in the short- and mid-term follow-up. Furthermore, our series provides additional evidence that the safety profile of long-term absorbable BSM is excellent with a very low rate of mesh-related complications even in a “keyhole” position encircling the abdominal esophagus. Nevertheless, the ideal technique for hiatus reconstruction has yet to be determined and—particularly with regard to the rapidly rising incidence of large HH in Western societies [43]—well-designed prospective and preferably randomized studies are needed to confirm long-term reliability of new-generation long-term absorbable BSM.
References
Kapischke M, Pries A (2014) Theodor Billroth’s vision and Karl Ziegler’s action: commemoration of the 40th day of death and the 50th anniversary of conferment of Nobel Prize for Chemistry of Karl Ziegler. Surgery 155(2):347–349
Geoffrion R, Larouche M (2021) Guideline No. 413: surgical management of apical pelvic organ prolapse in women. J Obstet Gynaecol Can 43(4):511–231
Henriksen NA, Montgomery A, Kaufmann R, Berrevoet F, East B, Fischer J et al (2020) Guidelines for treatment of umbilical and epigastric hernias from the European Hernia Society and Americas Hernia Society. Br J Surg 107(3):171–190
Hernandez-Granados P, Henriksen NA, Berrevoet F, Cuccurullo D, Lopez-Cano M, Nienhuijs S et al (2021) European Hernia Society guidelines on management of rectus diastasis. Br J Surg 108(10):1189–1191
HerniaSurge G (2018) International guidelines for groin hernia management. Hernia 22(1):1–165
Parker SG, Halligan S, Berrevoet F, de Beaux AC, East B, Eker HH et al (2021) Reporting guideline for interventional trials of primary and incisional ventral hernia repair. Br J Surg 108(9):1050–1055
Khajanchee YS, O’Rourke R, Cassera MA, Gatta P, Hansen PD, Swanstrom LL (2007) Laparoscopic reintervention for failed antireflux surgery: subjective and objective outcomes in 176 consecutive patients. Arch Surg 142(8):785–901 (discussion 791-2)
Muller-Stich BP, Kenngott HG, Gondan M, Stock C, Linke GR, Fritz F et al (2015) Use of mesh in laparoscopic paraesophageal hernia repair: a meta-analysis and risk-benefit analysis. PLoS ONE 10(10):e0139547
Spiro C, Quarmby N, Gananadha S (2020) Mesh-related complications in paraoesophageal repair: a systematic review. Surg Endosc 34(10):4257–4280
Li J, Cheng T (2019) Mesh erosion after hiatal hernia repair: the tip of the iceberg? Hernia 23(6):1243–1252
Cole WC, Balent EM, Masella PC, Kajiura LN, Matsumoto KW, Pierce LM (2015) An experimental comparison of the effects of bacterial colonization on biologic and synthetic meshes. Hernia 19(2):197–205
Xu X, Zhan M, Li X, Chen T, Yang L (2021) In vivo analysis of the resistance of the meshes to Escherichia coli infection. Front Surg 8:644227
Oelschlager BK, Pellegrini CA, Hunter JG, Brunt ML, Soper NJ, Sheppard BC et al (2011) Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 213(4):461–468
Jenkins ED, Yip M, Melman L, Frisella MM, Matthews BD (2010) Informed consent: cultural and religious issues associated with the use of allogeneic and xenogeneic mesh products. J Am Coll Surg 210(4):402–410
Williams SF, Rizk S, Martin DP (2013) Poly-4-hydroxybutyrate (P4HB): a new generation of resorbable medical devices for tissue repair and regeneration. Biomed Tech (Berl) 58(5):439–452
Martin DP, Badhwar A, Shah DV, Rizk S, Eldridge SN, Gagne DH et al (2013) Characterization of poly-4-hydroxybutyrate mesh for hernia repair applications. J Surg Res 184(2):766–773
Abdelmoaty WF, Dunst CM, Filicori F, Zihni AM, Davila-Bradley D, Reavis KM et al (2020) Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg 24(7):1477–1481
Panici Tonucci T, Asti E, Sironi A, Ferrari D, Bonavina L (2020) Safety and efficacy of crura augmentation with phasix ST mesh for large hiatal hernia: 3-year single-center experience. J Laparoendosc Adv Surg Tech A 30(4):369–372
Konstantinidis H, Charisis C (2022) Surgical treatment of large and complicated hiatal hernias with the new resorbable mesh with hydrogel barrier (Phasix ST): a preliminary study. J Robot Surg. https://doi.org/10.1007/s11701-022-01406-9
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258(1):1–7
Rajkomar K, Berney CR (2022) Large hiatus hernia: time for a paradigm shift? BMC Surg 22(1):264
Frantzides CT, Madan AK, Carlson MA, Stavropoulos GP (2002) A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg 137(6):649–652
Granderath FA, Schweiger UM, Kamolz T, Asche KU, Pointner R (2005) Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg 140(1):40–48
Ilyashenko VV, Grubnyk VV, Grubnik VV (2018) Laparoscopic management of large hiatal hernia: mesh method with the use of ProGrip mesh versus standard crural repair. Surg Endosc 32(8):3592–3598
Oor JE, Roks DJ, Koetje JH, Broeders JA, van Westreenen HL, Nieuwenhuijs VB et al (2018) Randomized clinical trial comparing laparoscopic hiatal hernia repair using sutures versus sutures reinforced with non-absorbable mesh. Surg Endosc 32(11):4579–4589
Watson DI, Thompson SK, Devitt PG, Aly A, Irvine T, Woods SD et al (2020) Five year follow-up of a randomized controlled trial of laparoscopic repair of very large hiatus hernia with sutures versus absorbable versus nonabsorbable mesh. Ann Surg 272(2):241–247
Analatos A, Hakanson BS, Lundell L, Lindblad M, Thorell A (2020) Tension-free mesh versus suture-alone cruroplasty in antireflux surgery: a randomized, double-blind clinical trial. Br J Surg 107(13):1731–1740
Petric J, Bright T, Liu DS, Wee Yun M, Watson DI (2022) Sutured versus mesh-augmented hiatus hernia repair: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 275(1):e45–e51
Sathasivam R, Bussa G, Viswanath Y, Obuobi RB, Gill T, Reddy A et al (2019) “Mesh hiatal hernioplasty” versus “suture cruroplasty” in laparoscopic para-oesophageal hernia surgery; a systematic review and meta-analysis. Asian J Surg 42(1):53–60
Memon MA, Siddaiah-Subramanya M, Yunus RM, Memon B, Khan S (2019) Suture cruroplasty versus mesh hiatal herniorrhaphy for large hiatal hernias (HHs): an updated meta-analysis and systematic review of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 29(4):221–232
Campos V, Palacio DS, Glina F, Tustumi F, Bernardo WM, Sousa AV (2020) Laparoscopic treatment of giant hiatal hernia with or without mesh reinforcement: a systematic review and meta-analysis. Int J Surg 77:97–104
Tam V, Winger DG, Nason KS (2016) A systematic review and meta-analysis of mesh vs suture cruroplasty in laparoscopic large hiatal hernia repair. Am J Surg 211(1):226–238
Gutschow CA (2022) Comment on “sutured versus mesh-augmented hiatus hernia repair: a systematic review and meta-analysis of randomized controlled trials” by Petric J, Bright T, Liu DS, et al. Ann Surg. 2022;275: e45–e51. Ann Surg Open 3(3):e201
Asti E, Lovece A, Bonavina L, Milito P, Sironi A, Bonitta G et al (2016) Laparoscopic management of large hiatus hernia: five-year cohort study and comparison of mesh-augmented versus standard crura repair. Surg Endosc 30(12):5404–5409
Zaninotto G, Portale G, Costantini M, Fiamingo P, Rampado S, Guirroli E et al (2007) Objective follow-up after laparoscopic repair of large type III hiatal hernia. Assessment of safety and durability. World J Surg 31(11):2177–2183
Saad AR, Velanovich V (2020) Anatomic observation of recurrent hiatal hernia: recurrence or disease progression? J Am Coll Surg 230(6):999–1007
Suppiah A, Sirimanna P, Vivian SJ, O’Donnell H, Lee G, Falk GL (2017) Temporal patterns of hiatus hernia recurrence and hiatal failure: quality of life and recurrence after revision surgery. Dis Esophagus 30(4):1–8
Linnaus ME, Garren A, Gould JC (2022) Anatomic location and mechanism of hiatal hernia recurrence: a video-based assessment. Surg Endosc 36(7):5451–5455
Velanovich V, Saad AR (2020) Toward a unified theory of occurrence and recurrence of hiatal hernia. Surgery 168(6):1170–1173
Keville S, Rabach L, Saad AR, Montera B, Velanovich V (2020) Evolution from the U-shaped to keyhole-shaped mesh configuration in the repair of paraesophageal and recurrent hiatal hernia. Surg Laparosc Endosc Percutan Tech 30(4):339–344
Oelschlager BK, Pellegrini CA, Hunter J, Soper N, Brunt M, Sheppard B et al (2006) Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg 244(4):481–490
Watson DI (2019) Current state of repair of large hiatal hernia. Int J Abdom Wall Hernia Surg 2(2):39–43
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Christian A. Gutschow received consulting fees and/or honoraria from Medtronic, B. Braun, BD/Bard medical and Micro-Tech Europe GmbH, and third party funding for his institution from B.Braun. Kristjan Ukegjini, Diana Vetter and Valerian Dirr have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ukegjini, K., Vetter, D., Dirr, V. et al. Hiatus hernia repair with a new-generation biosynthetic mesh: a 4-year single-center experience. Surg Endosc 37, 5295–5302 (2023). https://doi.org/10.1007/s00464-023-10005-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10005-0