Abstract
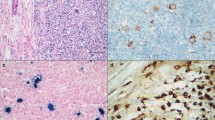
Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is a subtype of Hodgkin lymphoma that frequently shows a nodal growth pattern with abundant reactive B cells in the microenvironment. Early NLPHL cases can be particularly difficult to differentiate from progressively transformed germinal centers (PTGC). Since PTGC have been described to be IgG4 associated in a relatively high proportion of cases, the aim of the present study was to determine if IgG4 immunostaining can be helpful in the differential diagnosis between NLPHL and PTGC. We furthermore aimed to learn if LP cells can express IgG4. For this purpose, 58 cases of PTGC and 56 cases of NLPHL were assessed using IgG4 immunostaining. We could confirm that a significant number of PTGC cases showed high numbers of IgG4-positive plasma cells (22/58, 38%), whereas hot spot areas of IgG4-positive plasma cells were not found in any of the NLPHL cases. In lymph node areas with the differential diagnosis of NLPHL and PTGC, IgG4 immunostaining can therefore provide a helpful diagnostic tool to rule out NLPHL when a high number of IgG4-positive plasma cells are encountered. We also assessed 13 cases with a combination of NLPHL and PTGC in the same lymph node. Five of these cases presented hot spot areas of IgG4-positive plasma cells in the PTGC regions, while no significant numbers of IgG4-positive plasma cells were observed in the NLPHL part of the lymph node. LP cells were never IgG4 positive. Furthermore, immunoglobulin heavy chain rearrangements of single IgG4-positive plasma cells were analyzed, revealing a polyclonal plasma cell population. In summary, our data suggest that IgG4 immunostaining can provide additional information in the diagnostic workup of cases with the differential diagnosis of NLPHL and PTGC. IgG4’s inefficiency in clearing antigens may explain why lymph nodes with PTGC are usually strongly enlarged and develop a high number of hyperplastic germinal centers. Polyclonal immunoglobulin heavy chain rearrangements in IgG4-positive plasma cells further support the hypothesis that PTGC represent a misled immune reaction.

Similar content being viewed by others
References
Anagnostopoulos I, Hansmann ML, Franssila K, Harris M, Harris NL, Jaffe ES, Han J, van Krieken J, Poppema S, Marafioti T, Franklin J, Sextro M, Diehl V, Stein H (2000) European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood 96(5):1889–1899
Braeuninger A, Küppers R, Strickler JG, Wacker HH, Rajewsky K, Hansmann ML (1997) Hodgkin and Reed-Sternberg cells in lymphocyte predominant Hodgkin disease represent clonal populations of germinal center-derived tumor B cells. Proc Natl Acad Sci U S A 94(17):9337–9342
Schmid C, Sargent C, Isaacson PG (1991) L and H cells of nodular lymphocyte predominant Hodgkin’s disease show immunoglobulin light-chain restriction. Am J Pathol 139(6):1281–1289
Prakash S, Fountaine T, Raffeld M, Jaffe ES, Pittaluga S (2006) IgD positive L&H cells identify a unique subset of nodular lymphocyte predominant Hodgkin lymphoma. Am J Surg Pathol 30(5):585–592. https://doi.org/10.1097/01.pas.0000194741.87798.45
Hansmann ML, Godde-Salz E, Hui PK, Muller-Hermelink HK, Lennert K (1986) Cytogenetic findings in nodular paragranuloma (Hodgkin’s disease with lymphocytic predominance; nodular) and in progressively transformed germinal centers. Cancer Genet Cytogenet 21(4):319–325
Hansmann ML, Fellbaum C, Hui PK, Moubayed P (1990) Progressive transformation of germinal centers with and without association to Hodgkin’s disease. Am J Clin Pathol 93(2):219–226
Osborne BM, Butler JJ, Gresik MV (1992) Progressive transformation of germinal centers: comparison of 23 pediatric patients to the adult population. Mod Pathol 5(2):135–140
Bräuninger A, Yang W, Wacker HH, Rajewsky K, Küppers R, Hansmann ML (2001) B-cell development in progressively transformed germinal centers: similarities and differences compared with classical germinal centers and lymphocyte-predominant Hodgkin disease. Blood 97(3):714–719
Sato Y, Inoue D, Asano N, Takata K, Asaoku H, Maeda Y, Morito T, Okumura H, Ishizawa S, Matsui S, Miyazono T, Takeuchi T, Kuroda N, Orita Y, Takagawa K, Kojima M, Yoshino T (2012) Association between IgG4-related disease and progressively transformed germinal centers of lymph nodes. Mod Pathol 25(7):956–967. https://doi.org/10.1038/modpathol.2012.54
Hartmann S, Winkelmann R, Metcalf RA, Treetipsatit J, Warnke RA, Natkunam Y, Hansmann ML (2015) Immunoarchitectural patterns of progressive transformation of germinal centers with and without nodular lymphocyte-predominant Hodgkin lymphoma. Hum Pathol 46(11):1655–1661. https://doi.org/10.1016/j.humpath.2015.07.006
Swerdlow SH, Campo E, Harris NL, Jaffee ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2017
Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T et al (2012) Consensus statement on the pathology of IgG4-related disease. Mod Pathol 25(9):1181–1192. https://doi.org/10.1038/modpathol.2012.72
Küppers R, Zhao M, Hansmann ML, Rajewsky K (1993) Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J 12(13):4955–4967
Küppers R, Schneider M, Hansmann ML (2013) Laser-based microdissection of single cells from tissue sections and PCR analysis of rearranged immunoglobulin genes from isolated normal and malignant human B cells. Methods Mol Biol 971:49–63. https://doi.org/10.1007/978-1-62703-269-8_3
Bräuninger A, Küppers R, Spieker T, Siebert R, Strickler JG, Schlegelberger B, Rajewsky K, Hansmann ML (1999) Molecular analysis of single B cells from T cell-rich B-cell lymphoma shows the derivation of the tumor cells from mutating germinal center B cells and exemplifies means by which immunoglobulin genes are modified in germinal center B cells. Blood 93:2679–2687
Della-Torre E, Lanzillotta M, Doglioni C (2015) Immunology of IgG4-related disease. Clin Exp Immunol 181(2):191–206. https://doi.org/10.1111/cei.12641 PubMed PMID: 25865251; PubMed Central PMCID: PMCPMC4516435
van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E et al (2007) Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317(5844):1554–1557. https://doi.org/10.1126/science.1144603
Bindon CI, Hale G, Bruggemann M, Waldmann H (1988) Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med 168(1):127–142 PubMed PMID: 3260935; PubMed Central PMCID: PMCPMC2188986
Aalberse RC, Schuurman J (2002) IgG4 breaking the rules. Immunology 105(1):9–19 PubMed PMID: 11849310; PubMed Central PMCID: PMCPMC1782638
Go H, Kim JE, Kim YA, Chung HK, Khwarg SI, Kim CW, Jeon YK (2012) Ocular adnexal IgG4-related disease: comparative analysis with mucosa-associated lymphoid tissue lymphoma and other chronic inflammatory conditions. Histopathology 60(2):296–312. https://doi.org/10.1111/j.1365-2559.2011.04089.x
De Souza A, Ferry JA, Burghart DR, Tinguely M, Goyal A, Duncan LM, et al. IgG4 expression in primary cutaneous marginal zone lymphoma: a multicenter study. Appl Immunohistochem Mol Morphol. 2017. https://doi.org/10.1097/PAI.0000000000000462
Tsuboi H, Matsuo N, Iizuka M, Tsuzuki S, Kondo Y, Tanaka A, Moriyama M, Matsumoto I, Nakamura S, Sumida T (2012) Analysis of IgG4 class switch-related molecules in IgG4-related disease. Arthritis Res Ther 14(4):R171. https://doi.org/10.1186/ar3924 PubMed PMID: 22824292; PubMed Central PMCID: PMCPMC3580565
Carbonnelle-Puscian A, Copie-Bergman C, Baia M, Martin-Garcia N, Allory Y, Haioun C, Crémades A, Abd-Alsamad I, Farcet JP, Gaulard P, Castellano F, Molinier-Frenkel V (2009) The novel immunosuppressive enzyme IL4I1 is expressed by neoplastic cells of several B-cell lymphomas and by tumor-associated macrophages. Leukemia 23(5):952–960. https://doi.org/10.1038/leu.2008.380 PubMed PMID: 19436310; PubMed Central PMCID: PMCPMC2738850
Funding
Deutsche Forschungsgemeinschaft grant numbers HA6145/1-2 and HA6145/3-1
Author information
Authors and Affiliations
Contributions
KK: data acquisition and interpretation, drafting the manuscript; JB, BS, LT, MS: data acquisition and interpretation; MLH: contributed essential material, data interpretation; SH: design of the study, data interpretation, writing of the manuscript.
Corresponding author
Ethics declarations
The local ethics committee approved the study, and written informed consent from the donors was obtained in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kiil, K., Bein, J., Schuhmacher, B. et al. A high number of IgG4-positive plasma cells rules out nodular lymphocyte predominant Hodgkin lymphoma. Virchows Arch 473, 759–764 (2018). https://doi.org/10.1007/s00428-018-2460-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2460-8