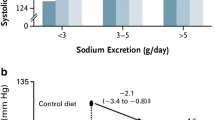
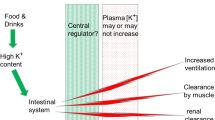
Abstract
Transmembrane potassium (K) gradients are key determinants of membrane potential that can modulate action potentials, control muscle contractility, and influence ion channel and transporter activity. Daily K intake is normally equal to the amount of K in the entire extracellular fluid (ECF) creating a critical challenge — how to maintain ECF [K] and membrane potential in a narrow range during feast and famine. Adaptations to maintain ECF [K] include sensing the K intake, sensing ECF [K] vs. desired set-point and activating mediators that regulate K distribution between ECF and ICF, and regulate renal K excretion. In this focused review, we discuss the basis of these adaptions, including (1) potential mechanisms for rapid feedforward signaling to kidney and muscle after a meal (before a rise in ECF [K]), (2) how skeletal muscles sense and respond to changes in ECF [K], (3) effects of K on aldosterone biosynthesis, and (4) how the kidney responds to changes in ECF [K] to modify K excretion. The concepts of sexual dimorphisms in renal K handling adaptation are introduced, and the molecular mechanisms that can account for the benefits of a K-rich diet to maintain cardiovascular health are discussed. Although the big picture of K homeostasis is becoming more clear, we also highlight significant pieces of the puzzle that remain to be solved, including knowledge gaps in our understanding of initiating signals, sensors and their connection to homeostatic adjustments of ECF [K].



Similar content being viewed by others
References
Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP (2013) Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 346:f1378
Amemiya M, Tabei K, Kusano E, Asano Y, Alpern RJ (1999) Incubation of OKP cells in low-K+ media increases NHE3 activity after early decrease in intracellular pH. Am J Physiol 276:C711–C716
Bandulik S, Tauber P, Lalli E, Barhanin J, Warth R (2015) Two-pore domain potassium channels in the adrenal cortex. Pflugers Arch 467:1027–1042
Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, Gonzalez-Rodriguez X, Vazquez N, Rodriguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, Garcia-Valdes J, Hadchouel J, Gamba G (2015) The effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26:1781–1786
Beggs MR, Young K, Pan W, O’Neill DD, Saurette M, Plain A, Rievaj J, Doschak MR, Cordat E, Dimke H, Alexander RT (2021) Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis. Proc Natl Acad Sci U S A 118
Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M (2013) Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 45:440–4, 444e1–2
Bia MJ, DeFronzo RA (1981) Extrarenal potassium homeostasis. Am J Physiol 240:F257–F268
Bia MJ, Tyler KA, DeFronzo RA (1982) Regulation of extrarenal potassium homeostasis by adrenal hormones in rats. Am J Physiol 242:F641–F644
Bignon Y, Pinelli L, Frachon N, Lahuna O, Figueres L, Houillier P, Lourdel S, Teulon J, Paulais M (2020) Defective bicarbonate reabsorption in Kir4.2 potassium channel deficient mice impairs acid-base balance and ammonia excretion. Kidney Int 97:304–315
Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, Van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R (2009) Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360:1960–70
Boyd-Shiwarski CR, Shiwarski DJ, Roy A, Namboodiri HN, Nkashama LJ, Xie J, McClain KL, Marciszyn A, Kleyman TR, Tan RJ, Stolz DB, Puthenveedu MA, Huang CL, Subramanya AR (2018) Potassium-regulated distal tubule WNK bodies are kidney-specific WNK1 dependent. Mol Biol Cell 29:499–509
Boyd-Shiwarski CR, Subramanya AR (2017) The renal response to potassium stress: integrating past with present. Curr Opin Nephrol Hypertens 26:411–418
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP (2012) Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482:98–102
Canonica J, Sergi C, Maillard M, Klusonova P, Odermatt A, Koesters R, Loffing-Cueni D, Loffing J, Rossier B, Frateschi S, Hummler E (2016) Adult nephron-specific MR-deficient mice develop a severe renal PHA-1 phenotype. Pflugers Arch 468:895–908
Cary R. Boyd-Shiwarski DJS, Shawn E. Griffiths, Rebecca T. Beacham, Logan Norrell, Daryl E. Morrison, Jun Wang, Jacob Mann, William Tennant, Eric N. Anderson, Jonathan Franks, Michael Calderon, Kelly A. Connolly, Claire J. Weaver, Claire C. Weckerly, Udai Bhan Pandey, Christopher J. Donnelly, Dandan Sun, Aylin R. Rodan, Arohan R. Subramanya (2022) WNK kinases sense molecular crowding and rescue cell volume via phase separation. bioRxiv 2022.01.10.475707
Castaneda-Bueno M, Ellison DH, Gamba G (2022) Molecular mechanisms for the modulation of blood pressure and potassium homeostasis by the distal convoluted tubule. EMBO Mol Med 14:e14273
Chadwick JA, Hauck JS, Lowe J, Shaw JJ, Guttridge DC, Gomez-Sanchez CE, Gomez-Sanchez EP, Rafael-Fortney JA (2015) Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J 29:4544–4554
Chaudhary P, Wainford RD (2020) Association of urinary sodium and potassium excretion with systolic blood pressure in the Dietary Approaches to Stop Hypertension Sodium Trial. J Hum Hypertens
Chen JC, Lo YF, Lin YW, Lin SH, Huang CL, Cheng CJ (2019) WNK4 kinase is a physiological intracellular chloride sensor. Proc Natl Acad Sci U S A
Chen P, Guzman JP, Leong PK, Yang LE, Perianayagam A, Babilonia E, Ho JS, Youn JH, Wang WH, McDonough AA (2006) Modest dietary K+ restriction provokes insulin resistance of cellular K+ uptake and phosphorylation of renal outer medulla K+ channel without fall in plasma K+ concentration. Am J Physiol Cell Physiol 290:C1355–C1363
Cheng L, Poulsen SB, Wu Q, Esteva-Font C, Olesen ETB, Peng L, Olde B, Leeb-Lundberg LMF, Pisitkun T, Rieg T, Dimke H, Fenton RA (2019) Rapid aldosterone-mediated signaling in the DCT increases activity of the thiazide-sensitive NaCl cotransporter. J Am Soc Nephrol 30:1454–1470
Choi CS, Lee FN, McDonough AA, Youn JH (2002) Independent regulation of in vivo insulin action on glucose versus K(+) uptake by dietary fat and K(+) content. Diabetes 51:915–920
Choi CS, Thompson CB, Leong PK, McDonough AA, Youn JH (2001) Short-term K(+) deprivation provokes insulin resistance of cellular K(+) uptake revealed with the K(+) clamp. Am J Physiol Renal Physiol 280:F95–F102
Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, Palmer BF, Cheung M, Wheeler DC, Winkelmayer WC, Pecoits-Filho R, Conference P (2019) Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int
Clausen T (2013) Quantification of Na+, K+ pumps and their transport rate in skeletal muscle: functional significance. J Gen Physiol 142:327–345
Cole TJ, Young MJ (2017) 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor null mice: informing cell-type-specific roles. J Endocrinol 234:T83–T92
Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, Yang CL, Ellison DH, Wang WH (2017) Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28:1814–1825
Czogalla J, Vohra T, Penton D, Kirschmann M, Craigie E, Loffing J (2016) The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflugers Arch 468:849–858
de Souza AMA, West CA (2018) Adaptive remodeling of renal Na+ and K+ transport during pregnancy. Curr Opin Nephrol Hypertens 27:379–383
DiFranco M, Hakimjavadi H, Lingrel JB, Heiny JA (2015) Na, K-ATPase alpha2 activity in mammalian skeletal muscle T-tubules is acutely stimulated by extracellular K+. J Gen Physiol 146:281–294
DiFranco M, Yu C, Quinonez M, Vergara JL (2015) Inward rectifier potassium currents in mammalian skeletal muscle fibres. J Physiol 593:1213–1238
Ellison DH, Velazquez H, Wright FS (1987) Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol 253:F546–F554
Faulkner JL, Belin de Chantemele EJ (2020) Female sex, a major risk factor for salt-sensitive hypertension. Curr Hypertens Rep 22:99
Ferraro PM, Mandel EI, Curhan GC, Gambaro G, Taylor EN (2016) Dietary protein and potassium, diet-dependent net acid load, and risk of incident kidney stones. Clin J Am Soc Nephrol 11:1834–1844
Filippini T, Naska A, Kasdagli MI, Torres D, Lopes C, Carvalho C, Moreira P, Malavolti M, Orsini N, Whelton PK, Vinceti M (2020) Potassium intake and blood pressure: a dose-response meta-analysis of randomized controlled trials. J Am Heart Assoc 9:e015719
Filippini T, Violi F, D’Amico R, Vinceti M (2017) The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol 230:127–135
Fodstad H, Gonzalez-Rodriguez E, Bron S, Gaeggeler H, Guisan B, Rossier BC, Horisberger JD (2009) Effects of mineralocorticoid and K+ concentration on K+ secretion and ROMK channel expression in a mouse cortical collecting duct cell line. Am J Physiol Renal Physiol 296:F966–F975
Frindt G, Houde V, Palmer LG (2011) Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol 301:F14-20
Frindt G, Palmer LG (2010) Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299:F890–F897
Frindt G, Yang L, Uchida S, Weinstein AM, Palmer LG (2017) Responses of distal nephron Na(+) transporters to acute volume depletion and hyperkalemia. Am J Physiol Renal Physiol 313:F62–F73
Funder J (2017) 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor activation and specificity-conferring mechanisms: a brief history. J Endocrinol 234:T17–T21
Glover M, Mercier Zuber A, Figg N, O’Shaughnessy KM (2010) The activity of the thiazide-sensitive Na(+)-Cl(-) cotransporter is regulated by protein phosphatase PP4. Can J Physiol Pharmacol 88:986–995
Greenlee M, Wingo CS, McDonough AA, Youn JH, Kone BC (2009) Narrative review: evolving concepts in potassium homeostasis and hypokalemia. Ann Intern Med 150:619–625
Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA (2012) SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287:37673–37690
Gruber S, Beuschlein F (2020) Hypokalemia and the prevalence of primary aldosteronism. Horm Metab Res 52:347–356
Gumz ML, Rabinowitz L, Wingo CS (2015) An integrated view of potassium homeostasis. N Engl J Med 373:60–72
Hattangady NG, Olala LO, Bollag WB, Rainey WE (2012) Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol 350:151–162
Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R, Reichold M, Tegtmeier I, Bendahhou S, Gomez-Sanchez CE, Aller MI, Wisden W, Weber A, Lesage F, Warth R, Barhanin J (2008) Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J 27:179–187
Hene RJ, Koomans HA, Rabelink AJ, Boer P, Dorhout Mees EJ (1988) Mineralocorticoid activity and the excretion of an oral potassium load in normal man. Kidney Int 34:697–703
Hennings JC, Andrini O, Picard N, Paulais M, Huebner AK, Cayuqueo IK, Bignon Y, Keck M, Corniere N, Bohm D, Jentsch TJ, Chambrey R, Teulon J, Hubner CA, Eladari D (2017) The ClC-K2 chloride channel is critical for salt handling in the distal nephron. J Am Soc Nephrol 28:209–217
Hoorn EJ, Gritter M, Cuevas CA, Fenton RA (2020) Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev 100:321–356
Hoorn EJ, Zietse R (2015) Gut-kidney kaliuretic signaling: looking forward to feeding. Kidney Int 88:1230–1232
Hu R, McDonough AA, Layton AT (2020) Sex differences in solute transport along the nephrons: effects of Na(+) transport inhibition. Am J Physiol Renal Physiol 319:F487–F505
Ishizawa K, Wang Q, Li J, Yamazaki O, Tamura Y, Fujigaki Y, Uchida S, Lifton RP, Shibata S (2019) Calcineurin dephosphorylates Kelch-like 3, reversing phosphorylation by angiotensin II and regulating renal electrolyte handling. Proc Natl Acad Sci U S A 116:3155–3160
Ishizawa K, Xu N, Loffing J, Lifton RP, Fujita T, Uchida S, Shibata S (2016) Potassium depletion stimulates Na-Cl cotransporter via phosphorylation and inactivation of the ubiquitin ligase Kelch-like 3. Biochem Biophys Res Commun 480:745–751
Jensen IS, Larsen CK, Leipziger J, Sorensen MV (2016) Na(+) dependence of K(+) -induced natriuresis, kaliuresis and Na(+) /Cl(-) cotransporter dephosphorylation. Acta Physiol (Oxf) 218:49–61
Kamel KS, Schreiber M, Halperin ML (2014) Integration of the response to a dietary potassium load: a paleolithic perspective. Nephrol Dial Transplant 29:982–989
Kirabo A (2017) A new paradigm of sodium regulation in inflammation and hypertension. Am J Physiol Regul Integr Comp Physiol 313:R706–R710
Kobayashi K, Uchida S, Mizutani S, Sasaki S, Marumo F (2001) Intrarenal and cellular localization of CLC-K2 protein in the mouse kidney. J Am Soc Nephrol 12:1327–1334
Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schofl C, Renz W, Santoro D, Niendorf T, Muller DN, Neininger M, Cavallaro A, Eckardt KU, Schmieder RE, Luft FC, Uder M, Titze J (2012) (23)Na magnetic resonance imaging of tissue sodium. Hypertension 59:167–172
Kortenoeven MLA, Cheng L, Wu Q, Fenton RA (2021) An in vivo protein landscape of the mouse DCT during high dietary K(+) or low dietary Na(+) intake. Am J Physiol Renal Physiol 320:F908–F921
Kortenoeven MLA, Esteva-Font C, Dimke H, Poulsen SB, Murali SK, Fenton RA (2021) High dietary potassium causes ubiquitin-dependent degradation of the kidney sodium-chloride cotransporter. J Biol Chem:100915
Krishna GG, Kapoor SC (1991) Potassium depletion exacerbates essential hypertension. Ann Intern Med 115:77–83
Li J, Xu S, Yang L, Yang J, Wang CJ, Weinstein AM, Palmer LG, Wang T (2019) Sex difference in kidney electrolyte transport II: impact of K(+) intake on thiazide-sensitive cation excretion in male and female mice. Am J Physiol Renal Physiol 317:F967–F977
Lindinger MI, Cairns SP (2021) Regulation of muscle potassium: exercise performance, fatigue and health implications. Eur J Appl Physiol 121:721–748
Lindinger MI, Franklin TW, Lands LC, Pedersen PK, Welsh DG (1985) Heigenhauser GJ (2000) NaHCO(3) and KHCO(3) ingestion rapidly increases renal electrolyte excretion in humans. J Appl Physiol 88:540–550
Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM (2011) Role of NKCC in BK channel-mediated net K(+) secretion in the CCD. Am J Physiol Renal Physiol 301:F1088–F1097
Lopez-Cayuqueo KI, Chavez-Canales M, Pillot A, Houillier P, Jayat M, Baraka-Vidot J, Trepiccione F, Baudrie V, Busst C, Soukaseum C, Kumai Y, Jeunemaitre X, Hadchouel J, Eladari D, Chambrey R (2018) A mouse model of pseudohypoaldosteronism type II reveals a novel mechanism of renal tubular acidosis. Kidney Int 94:514–523
Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, International Consortium for Blood P, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X (2012) KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44(456–60):S1-3
Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J (2002) An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538:391–404
McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH (2011) A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14:352–364
McDonough AA, Veiras LC, Guevara CA, Ralph DL (2017) Cardiovascular benefits associated with higher dietary K+ vs. lower dietary Na+: evidence from population and mechanistic studies. Am J Physiol Endocrinol Metab 312:E348–E356
McDonough AA, Youn JH (2017) Potassium homeostasis: the knowns, the unknowns, and the health benefits. Physiology (Bethesda) 32:100–111
McFarlin BE, Chen Y, Priver TS, Ralph DL, Mercado A, Gamba G, Madhur MS, McDonough AA (2020) Coordinate adaptations of skeletal muscle and kidney to maintain extracellular [K(+)] during K(+)-deficient diet. Am J Physiol Cell Physiol 319:C757–C770
McKenna MJ, Gissel H, Clausen T (2003) Effects of electrical stimulation and insulin on Na+-K+-ATPase ([3H]ouabain binding) in rat skeletal muscle. J Physiol 547:567–580
Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S, Investigators P (2014) Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371:601–611
Morita H, Fujiki N, Miyahara T, Lee K, Tanaka K (2000) Hepatoportal bumetanide-sensitive K(+)-sensor mechanism controls urinary K(+) excretion. Am J Physiol Regul Integr Comp Physiol 278:R1134–R1139
Mukherjee A, Yang CL, McCormick JA, Martz K, Sharma A, Ellison DH (2021) Roles of WNK4 and SPAK in K(+)-mediated dephosphorylation of the NaCl cotransporter. Am J Physiol Renal Physiol 320:F719–F733
Murali SK, Little R, Poulsen SB, Ferdaus MZ, Ellison DH, McCormick JA, Fenton RA (2021) Potassium effects on NCC are attenuated during inhibition of cullin E3-ubiquitin ligases. Cells 11
Murillo-de-Ozores AR, Rodriguez-Gama A, Carbajal-Contreras H, Gamba G, Castaneda-Bueno M (2021) WNK4 kinase: from structure to physiology. Am J Physiol Renal Physiol 320:F378–F403
Ndanuko RN, Ibrahim R, Hapsari RA, Neale EP, Raubenheimer D, Charlton KE (2021) Association between the urinary sodium to potassium ratio and blood pressure in adults: a systematic review and meta-analysis. Adv Nutr
Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, Zhang J, Tian M, Huang L, Li Z, Yu Y, Zhao Y, Zhou B, Sun J, Liu Y, Yin X, Hao Z, Yu J, Li KC, Zhang X, Duan P, Wang F, Ma B, Shi W, Di Tanna GL, Stepien S, Shan S, Pearson SA, Li N, Yan LL, Labarthe D, Elliott P (2021) Effect of salt substitution on cardiovascular events and death. N Engl J Med 385:1067–1077
Nesterov V, Bertog M, Korbmacher C (2022) High baseline ROMK activity in the mouse late distal convoluted and early connecting tubule probably contributes to aldosterone-independent K(+) secretion. Am J Physiol Renal Physiol 322:F42–F54
Nguyen MT, Yang LE, Fletcher NK, Lee DH, Kocinsky H, Bachmann S, Delpire E, McDonough AA (2012) Effects of K+-deficient diets with and without NaCl supplementation on Na+, K+, and H2O transporters’ abundance along the nephron. Am J Physiol Renal Physiol 303:F92-104
Nguyen TQ, Maalouf NM, Sakhaee K, Moe OW (2011) Comparison of insulin action on glucose versus potassium uptake in humans. Clin J Am Soc Nephrol 6:1533–1539
Nomura N, Shoda W, Wang Y, Mandai S, Furusho T, Takahashi D, Zeniya M, Sohara E, Rai T, Uchida S (2018) Role of ClC-K and barttin in low potassium-induced sodium chloride cotransporter activation and hypertension in mouse kidney. Biosci Rep 38
Oh KS, Oh YT, Kim SW, Kita T, Kang I, Youn JH (2011) Gut sensing of dietary K(+) intake increases renal K(+)excretion. Am J Physiol Regul Integr Comp Physiol 301:R421–R429
Oh YT, Kim J, Youn JH (2013) Role of pituitary in K+ homeostasis: impaired renal responses to altered K+ intake in hypophysectomized rats. Am J Physiol Regul Integr Comp Physiol 304:R1166–R1174
Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T (2013) The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J 451:111–122
Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G (2006) The Na+:Cl- cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281:28755–28763
Palygin O, Pochynyuk O, Staruschenko A (2017) Role and mechanisms of regulation of the basolateral Kir 4.1/Kir 5.1K(+) channels in the distal tubules. Acta Physiol (Oxf) 219:260–273
Pei L, Solis G, Nguyen MT, Kamat N, Magenheimer L, Zhuo M, Li J, Curry J, McDonough AA, Fields TA, Welch WJ, Yu AS (2016) Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. J Clin Invest 126:2509–2518
Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, Rajaram RD, Staub O, Bleich M, Schweda F, Loffing J (2016) Extracellular K(+) rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl(-) -dependent and independent mechanisms. J Physiol 594:6319–6331
Penton D, Moser S, Wengi A, Czogalla J, Rosenbaek LL, Rigendinger F, Faresse N, Martins JR, Fenton RA, Loffing-Cueni D, Loffing J (2019) Protein phosphatase 1 inhibitor-1 mediates the cAMP-dependent stimulation of the renal NaCl cotransporter. J Am Soc Nephrol 30:737–750
Penton D, Vohra T, Banki E, Wengi A, Weigert M, Forst AL, Bandulik S, Warth R, Loffing J (2020) Collecting system-specific deletion of Kcnj10 predisposes for thiazide- and low-potassium diet-induced hypokalemia. Kidney Int 97:1208–1218
Pham TD, Elengickal AJ, Verlander JW, Al-Qusairi L, Chen C, Abood DC, King SA, Loffing J, Welling PA, Wall SM (2022) Pendrin null mice develop severe hypokalemia following dietary K(+) restriction: role of ENaC. Am J Physiol Renal Physiol
Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ (2014) Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7:ra41
Picard N, Trompf K, Yang CL, Miller RL, Carrel M, Loffing-Cueni D, Fenton RA, Ellison DH, Loffing J (2014) Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol 25:511–522
Polidoro JZ, Luchi WM, Seguro AC, Malnic G, Girardi ACC (2022) Paracrine and endocrine regulation of renal K(+) secretion. Am J Physiol Renal Physiol 322:F360–F377
Poulsen SB, Cheng L, Penton D, Kortenoeven MLA, Matchkov VV, Loffing J, Little R, Murali SK, Fenton RA (2021) Activation of the kidney sodium chloride cotransporter by the beta2-adrenergic receptor agonist salbutamol increases blood pressure. Kidney Int 100:321–335
Poulsen SB, Fenton RA (2019) K(+) and the renin-angiotensin-aldosterone system: new insights into their role in blood pressure control and hypertension treatment. J Physiol 597:4451–4464
Preston RA, Afshartous D, Rodco R, Alonso AB, Garg D (2015) Evidence for a gastrointestinal-renal kaliuretic signaling axis in humans. Kidney Int 88:1383–1391
Rabinowitz L (1989) Homeostatic regulation of potassium excretion. J Hypertens 7:433–442
Rabinowitz L (1996) Aldosterone and potassium homeostasis. Kidney Int 49:1738–1742
Rao R, Bhalla V, Pastor-Soler NM (2019) Intercalated cells of the kidney collecting duct in kidney physiology. Semin Nephrol 39:353–367
Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA (2014) Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306:F1059–F1068
Richardson C, Alessi DR (2008) The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 121:3293–3304
Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H (2007) The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int 72:566–573
Rosenbaek LL, Assentoft M, Pedersen NB, MacAulay N, Fenton RA (2012) Characterization of a novel phosphorylation site in the sodium-chloride cotransporter, NCC. J Physiol 590:6121–6139
Rosenbaek LL, Rizzo F, MacAulay N, Staub O, Fenton RA (2017) Functional assessment of sodium chloride cotransporter NCC mutants in polarized mammalian epithelial cells. Am J Physiol Renal Physiol 313:F495–F504
Rossier BC, Baker ME, Studer RA (2015) Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev 95:297–340
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH, Group DA-SCR (2001) Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 344:3–10
Schlingmann KP, Renigunta A, Hoorn EJ, Forst A-L, Renigunta V, Atanasov V, Mahendran S, Barakat TS, Gillion V, Godefroid N, Brooks AS, Lugtenberg D, Lake J, Debaix H, Rudin C, Knebelmann B, Tellier S, Rousset-Rouvière C, Viering D, de Baaij JHF, Weber S, Palygin O, Staruschenko A, Kleta R, Houillier P, Bockenhauer D, Devuyst O, Vargas-Poussou R, Warth R, Zdebik AA, Konrad M (2021) Defects in KCNJ16 cause a novel tubulopathy with hypokalemia, salt wasting, disturbed acid-base homeostasis, and sensorineural deafness. J Am Soc Nephrol 32:1498–1512
Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP (2009) Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106:5842–5847
Scholl UI, Lifton RP (2013) New insights into aldosterone-producing adenomas and hereditary aldosteronism: mutations in the K+ channel KCNJ5. Curr Opin Nephrol Hypertens 22:141–147
Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC Jr (1994) Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 330:1776–1781
Shalomov B, Handklo-Jamal R, Reddy HP, Theodor N, Bera AK, Dascal N (2021) A revised mechanism of action of hyperaldosteronism-linked mutations in cytosolic domains of GIRK4 (KCNJ5). J Physiol
Shibata S (2017) 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor and NaCl transport mechanisms in the renal distal nephron. J Endocrinol 234:T35–T47
Shibata S, Arroyo JP, Castaneda-Bueno M, Puthumana J, Zhang J, Uchida S, Stone KL, Lam TT, Lifton RP (2014) Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci U S A 111:15556–15561
Shoda W, Nomura N, Ando F, Mori Y, Mori T, Sohara E, Rai T, Uchida S (2017) Calcineurin inhibitors block sodium-chloride cotransporter dephosphorylation in response to high potassium intake. Kidney Int 91:402–411
Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP (1996) Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12:24–30
Soleimani M, Bergman JA, Hosford MA, McKinney TD (1990) Potassium depletion increases luminal Na+/H+ exchange and basolateral Na+:CO3=:HCO3- cotransport in rat renal cortex. J Clin Invest 86:1076–1083
Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J (2013) Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int
Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J (2013) Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83:811–824
Sorensen MV, Saha B, Jensen IS, Wu P, Ayasse N, Gleason CE, Svendsen SL, Wang WH, Pearce D (2019) Potassium acts through mTOR to regulate its own secretion. JCI Insight 5
Su XT, Ellison DH, Wang WH (2019) Kir4.1/Kir5.1 in the DCT plays a role in the regulation of renal K(+) excretion. Am J Physiol Renal Physiol 316:F582–F586
Svendsen SL, Kornvig S, Berg P, Jensen IS, de Araujo I, Larsen CK, Leipziger J, Sorensen MV (2022) Dietary K(+) acts as a genuine diuretic. Acta Physiol (Oxf) 234:e13762
Takahashi D, Mori T, Nomura N, Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S (2014) WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep 34
Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH (2016) Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27:2436–2445
Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH (2015) Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21:39–50
Terry EE, Zhang X, Hoffmann C, Hughes LD, Lewis SA, Li J, Wallace MJ, Riley LA, Douglas CM, Gutierrez-Monreal MA, Lahens NF, Gong MC, Andrade F, Esser KA, Hughes ME (2018) Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. Elife 7
Thompson CB, Choi C, Youn JH, McDonough AA (1999) Temporal responses of oxidative vs. glycolytic skeletal muscles to K+ deprivation: Na+ pumps and cell cations. Am J Physiol 276:C1411–C1419
Thompson CB, McDonough AA (1996) Skeletal muscle Na, K-ATPase alpha and beta subunit protein levels respond to hypokalemic challenge with isoform and muscle type specificity. J Biol Chem 271:32653–32658
Thomson MN, Schneider W, Mutig K, Ellison DH, Kettritz R, Bachmann S (2018) Patients with hypokalemia develop WNK bodies in the distal convoluted tubule of the kidney. Am J Physiol Renal Physiol
Thomson MN, Schneider W, Mutig K, Ellison DH, Kettritz R, Bachmann S (2019) Patients with hypokalemia develop WNK bodies in the distal convoluted tubule of the kidney. Am J Physiol Renal Physiol 316:F292–F300
Titze J, Luft FC (2017) Speculations on salt and the genesis of arterial hypertension. Kidney Int 91:1324–1335
Tomilin V, Mamenko M, Zaika O, Wingo CS, Pochynyuk O (2019) TRPV4 deletion protects against hypokalemia during systemic K(+) deficiency. Am J Physiol Renal Physiol 316:F948–F956
Tomilin VN, Zaika O, Subramanya AR, Pochynyuk O (2018) Dietary K(+) and Cl(-) independently regulate basolateral conductance in principal and intercalated cells of the collecting duct. Pflugers Arch 470:339–353
Tsuchiya Y, Nakashima S, Banno Y, Suzuki Y, Morita H (2004) Effect of high-NaCl or high-KCl diet on hepatic Na+- and K+-receptor sensitivity and NKCC1 expression in rats. Am J Physiol Regul Integr Comp Physiol 286:R591–R596
Unwin R, Capasso G, Giebisch G (1994) Potassium and sodium transport along the loop of Henle: effects of altered dietary potassium intake. Kidney Int 46:1092–1099
Valinsky WC, Touyz RM, Shrier A (2018) Aldosterone, SGK1, and ion channels in the kidney. Clin Sci (Lond) 132:173–183
Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA (2017) Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28:3504–3517
Veiras LC, Han J, Ralph DL, McDonough AA (2016) Potassium supplementation prevents sodium chloride cotransporter stimulation during angiotensin II hypertension. Hypertension 68:904–912
Velazquez H, Ellison DH, Wright FS (1987) Chloride-dependent potassium secretion in early and late renal distal tubules. Am J Physiol 253:F555–F562
Vitari AC, Deak M, Morrice NA, Alessi DR (2005) The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391:17–24
Volkl H, Lang F (1991) Electrophysiology of ammonia transport in renal straight proximal tubules. Kidney Int 40:1082–1089
Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA (2011) Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300:F1385–F1393
Wang MX, Cuevas CA, Su XT, Wu P, Gao ZX, Lin DH, McCormick JA, Yang CL, Wang WH, Ellison DH (2018) Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 93:893–902
Wang WH (2016) Basolateral Kir4.1 activity in the distal convoluted tubule regulates K secretion by determining NaCl cotransporter activity. Curr Opin Nephrol Hypertens 25:429–435
Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA (2001) The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest 108:215–222
Webb TN, Carrisoza-Gaytan R, Montalbetti N, Rued A, Roy A, Socovich AM, Subramanya AR, Satlin LM, Kleyman TR, Carattino MD (2016) Cell-specific regulation of L-WNK1 by dietary K. Am J Physiol Renal Physiol 310:F15-26
Wei KY, Gritter M, Vogt L, de Borst MH, Rotmans JI, Hoorn EJ (2020) Dietary potassium and the kidney: lifesaving physiology. Clin Kidney J 13:952–968
Weinstein AM (1986) A mathematical model of the rat proximal tubule. Am J Physiol 250:F860–F873
Weinstein AM (2017) A mathematical model of the rat kidney: K(+)-induced natriuresis. Am J Physiol Renal Physiol 312:F925–F950
Weinstein AM (2022) A mathematical model of the rat kidney. IV. Whole kidney response to hyperkalemia. Am J Physiol Renal Physiol 322:F225–F244
Wen D, Cornelius RJ, Yuan Y, Sansom SC (2013) Regulation of BK-alpha expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol 305:F463–F476
West CA, Welling PA, West DA Jr, Coleman RA, Cheng KY, Chen C, DuBose TD Jr, Verlander JW, Baylis C, Gumz ML (2018) Renal and colonic potassium transporters in the pregnant rat. Am J Physiol Renal Physiol 314:F251–F259
Woda CB, Bragin A, Kleyman TR, Satlin LM (2001) Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280:F786–F793
Wright FS, Strieder N, Fowler NB, Giebisch G (1971) Potassium secretion by distal tubule after potassium adaptation. Am J Physiol 221:437–448
Wu P, Gao ZX, Zhang DD, Su XT, Wang WH, Lin DH (2019) Deletion of Kir5.1 impairs renal ability to excrete potassium during increased dietary potassium intake. J Am Soc Nephrol 30:1425–1438
Wu P, Su XT, Gao ZX, Zhang DD, Duan XP, Xiao Y, Staub O, Wang WH, Lin DH (2020) Renal tubule Nedd4-2 deficiency stimulates Kir4.1/Kir5.1 and thiazide-sensitive NaCl cotransporter in distal convoluted tubule. J Am Soc Nephrol 31:1226–1242
Xu S, Li J, Yang L, Wang CJ, Liu T, Weinstein AM, Palmer LG, Wang T (2021) Sex difference in kidney electrolyte transport III: Impact of low K intake on thiazide-sensitive cation excretion in male and female mice. Pflugers Arch 473:1749–1760
Yang L, Xu Y, Gravotta D, Frindt G, Weinstein AM, Palmer LG (2021) ENaC and ROMK channels in the connecting tubule regulate renal K+ secretion. J Gen Physiol 153
Youn JH (2013) Gut sensing of potassium intake and its role in potassium homeostasis. Semin Nephrol 33:248–256
Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH (2014) KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci U S A 111:11864–11869
Acknowledgements
The authors thank Dr. Jang H. Youn for the careful reading and suggestions.
Funding
DK123780 and DK132613-01 to AM. Leducq Foundation (17CVD05), the Novo Nordisk Foundation (NNF21OC0067647, NNF17OC0029724, NNF19OC0058439), and the Independent Research Fund Denmark to RAF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McDonough, A.A., Fenton, R.A. Potassium homeostasis: sensors, mediators, and targets. Pflugers Arch - Eur J Physiol 474, 853–867 (2022). https://doi.org/10.1007/s00424-022-02718-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-022-02718-3