Abstract
Purpose
The study aimed to determine the incidence and long-term evolution of COVID-related olfactory (OD) and gustatory (GD) dysfunction, the recovery timeline, and the association with other symptoms. The secondary objective was to identify the predictive clinical factors for the evolution of these symptoms.
Methods
A prospective case–control study was conducted from March 15 to October 15, 2020, in health workers with COVID-19 related symptoms in a tertiary care hospital. 320 patients were included after 6 months of follow-up: 195 in the case group and 125 in the control group. Olfactory dysfunction (OD), dysosmia, and gustatory dysfunction (GD) onset and recovery rate after 6 months follow-up are analyzed in both groups.
Results
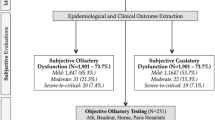
There were 125 (64.1%) in case group patients with OD and 118 (60.5%) with GD. Total or partial recovery OD and GD was found in 89%, mainly in the first 2 months. In the control group, there were 14 (11.2%) patients with OD and 33 (26.4%) patients with GD, with 100% of total/partial recovery.
Conclusion
In both groups, OD and GD showed high-resolution rates during the first two months after the onset of symptoms. Nevertheless, 11% of the case group patients did not show any recovery, and the partial resolution was present in 30% of our patients, at the 6 months follow-up. We found a high correlation between OD and GD, both in the appearance of symptoms and in their recovery. Nasal obstruction and dyspnea have been identified as risk factors for the persistence of symptoms.
Similar content being viewed by others
Introduction
Hyposmia has proven to be one of the most prevalent symptoms of COVID, despite not being recognized as the main symptom at the beginning of the pandemic, different reports show a high prevalence of olfactory (OD) and taste (GD) dysfunction, especially in western countries [1].
The upper respiratory tract plays a key role in the physiopathology of viral infections since it is the most common route of entrance and the area where the initial viral replication and colonization takes place [2]. Along with sinonasal pathology, trauma, or neurodegenerative diseases, viral infections are one of the most frequent causes of hyposmia in patients requiring ENT consultation [3, 4].
The SARS-CoV-2 infects human cells through the angiotensin-converting enzyme (ACE-2) receptor that is broadly expressed in the nasal/oral mucosa, as well as in the sustentacular cells of the olfactory epithelium (SUS) [5]. Moreover, according to other studies, SARS-CoV is demonstrating a strong neurotropism for the olfactory bulb cells, especially in the initial stages of the disease [6].
OD is described as an early symptom of SARS-CoV-2 infection by several studies. Furthermore, OD and GD seem to show a higher prevalence in women, young people, and healthy patients with mild disease [7, 8]. It is also remarkable, that hyposmia is the initial symptom in several cases (27%) and could be an isolated manifestation of the illness [9].
The reported prevalence of gustatory dysfunction in COVID-19 infection is 43.93–56.4% [1, 10]. Taste Dysfunction may also represent an early symptom suggestive of infection, but it remains unclear as to whether GD exists in COVID-19, or is a loss of retronasal smell [11]. This fact is due to the paucity of studies based on objective evidence for the measurement of GD since the reported prevalence of GD is mainly based on self-reported data [12].
ACE2 receptors have been identified in humans with high expression levels in the tongue [13]. In this sense, ACE2 receptors could have a key role in the development of taste dysfunction in COVID-19 patients [14]. On the other hand, central lesions could be another source of gustatory dysfunction in these patients due to many overlapping brain areas between the taste and olfactory systems [15].
Studies related to OD and GD in COVID patients describe high recovery rates within the first weeks, therefore no specific treatment or complementary test is recommended [16]. However, there are few studies of the long-term evolution regarding OD and GD in COVID patients beyond the first weeks [4, 17]. Likewise, no clear data is available on the association between OD and GD [11].
The present study analyzes the long-term evolution of OD and GD of health care workers with or without COVID, the recovery time, the predictive factor for improvement, as well as the association between both symptoms.
Materials and methods
We performed a prospective case–control study from March 15 to October 15, 2020, recruiting a group of health workers (physicians, nurses, assistants, cleaning workers, administrative workers) from a tertiary care hospital center with a high incidence of COVID between March 15 and April 7. The institutional review board, CEIM University Hospital of Getafe (CV20/24), approved this project. Patient informed consent was obtained from every patient.
RT-PCR (reverse transcriptase-polymerase chain reaction) methods detected the presence of COVID-19 in respiratory specimens, from nasal and pharyngeal swabs. Allplex™ 2019—nCoV Assay (Seegene) is a multiplex real-time PCR assay for simultaneous detection of 3 target genes of SARS-CoV-2 in a single tube. The assay is designed to detect RdRP, N genes specific for SARS-CoV-2, and E gene for all Sarbecovirus including SARS-CoV-2.
The inclusion criteria of the study were:
-
Signed written informed consent for participation in the study
-
Over 18 years of age
-
COVID-19 related symptoms
-
PCR performed from nasal-pharyngeal swab for SARS-CoV-2
-
Hospital worker at the time of diagnosis
-
At least 6 months of follow-up
The exclusion criteria were as follows:
-
History of nasal surgery, sinusitis, chronic rhinitis, polyposis, olfaction, and/or gustatory disorders
-
Neurodegenerative disease
-
A medical or psychological condition that limits the study
Patients with a positive RT-PCR at the time of inclusion were considered cases, and those with a negative RT-PCR were considered control. An Otolaryngologist at the onset of symptoms carries out a personal consultation. Anamnesis, physical examination, and a collection of nasopharyngeal swabs were performed. The anamnesis focused especially on the presence of OD/GD (onset, lasting, regression), nasal symptoms, other chemo-sensitive symptoms (dysosmia, dysgeusia, etc.), and the most prevalent COVID symptoms (fever, cough, dyspnea, myalgia, etc.). We consider GD a reduced or absent taste (sweet, salty, acid, bitter, umami), as well as the alteration in the quality of taste (parageusia), both with solid and liquid foods, during the study time. On the other hand, we consider OD a reduced or absent perception of odors as well as the alteration in the quality of smell (disosmia). In addition, clinical variables, such as diabetes mellitus, hypertension, or dyslipidemia were collected.
Based on previous studies of self-assessment of smell and creation of different categories depending on the level of the dysfunction [19], Visual Analog Scales (VAS) were used, ranging from 0 (no perception) to 10 (excellent perception). According to the VAS scale, we categorized the quantitative variables of hyposmia and hypogeusia in 3 intervals: 0–3 (severe), 4–7 (moderate), and 8–10 (mild). We have considered the recovery of symptoms when the VAS score moves from severe to moderate/mild or moderate to mild. Complete recovery of symptoms is only considered when mild VAS is achieved, and partial recovery is defined when mild VAS is not reached. When none of these situations takes place, non-recovery is considered.
Those positive RT-PCR patients continued regular reviews every 2 weeks until the PCR test was negative. Subsequently, the study was completed by a serological immunoglobulin G antibodies (IgG) test, 2 months after the PCR test in both groups. The monitoring of patients was done through monthly, quarterly, and semi-annual telephone consultations. In these reviews, we ask the patients about OD/GD VAS and the development of chemo-sensitive symptoms.
No empirical treatment for hyposmia or hypogeusia was indicated in any patient during the study.
Statistical analysis
Statistical Analysis Statistical Package for the Social Sciences for Windows (SPSS version 25.0; IBM Corp, Armonk, NY, USA) was used to perform the statistical analyses. The potential associations between epidemiological, clinical and olfactory, and gustatory outcomes have been assessed through cross-tab generation between two variables (binary or categorical variables) and the Chi-square test. Categorical variables between both age and sex-matched groups were summarized by counts and frequencies and compared using odds ratio (OR) with 95% CIs. The relationship between VAS variables was investigated using Spearman’s correlation coefficient.
The normally distributed variables were presented with means and SDs.
A Kaplan–Meier time-to-event method was used to calculate the percentage of subjects who had a total recovery from their OD. Additionally, separate curves were constructed for the two different groups, case, and controls, created based on the results of the RT-PCR. The log-rank test was used to compare the different survival distributions.
Results
A total of 355 patients with suspected COVID met the inclusion criteria and were recruited. 320 (90.1%) completed the 6-month follow-up and were included in the final case–control study. Of these, 195 (61%) patients with positive RT-PCR and 125 (39%) with negative RT-PCR, as part of the case and control group, respectively. Table 1 shows the demographic characteristics of the sample in the case group and control group, with no significant differences in baseline between groups.
In the 6-month follow-up, 14 patients (4.4%) required hospital admission, all of them belonged to the case group. No patient required intensive care. There were no deaths during the study.
Table 2 shows the incidence of symptoms studied in our sample. OD and GD were described in 125 (64.1%) and 118 (60.5%) in case group patients, respectively, and 14 (11.2%) with OD and 33 (26.4%) with GD in control group patients.
The presence of certain symptoms such as hyposmia (OR 13.89), hypogeusia (OR 17.6), and dysosmia (OR 14.94) was strongly associated with a positive RT-PCR.
Patients who initially had hyposmia had 3.86 times higher odds of developing dysosmia if the RT-PCR was positive.
In case-group patients, OD was the first symptom in 11% of our population. The timing of the onset of hyposmia was coincident with other symptoms in 14% of the subjects. The mean time of OD onset concerning another symptom was 4.2 days (± 5.24). At the time of diagnosis, there was a significant positive association between OD and GD (p < 0.001), and their VAS was highly correlated (r2 0.714). There is also a significant correlation between the OD and GD recovery on the VAS scale (r2 0.684).
The average time for negative PCR was 23.4 days (± 9.8). OD recovered before negative PCR in 66 (53%) subjects. In the statistical analysis, there is no association between negative PCR and OD recovery.
IgG antibodies are detected in the serological study of 156 case group patients (80%). There is no statistically significant correlation between the development of antibodies and the severity or recovery of OD or GD in our series.
At the end of the study, 111 (88.8%) patients in our case group presented OD recovery, which was complete in 73 (58.4%) subjects, and partial in 38 (30.4%) cases. Fourteen patients (11.2%) do not show recovery (Table 3).
According to the Kaplan–Meier method, most of the OD recovery (partial or complete) takes place in the first 2 months with the stability of symptoms afterward in both groups. Considering the control group, 100% of the patients present a total or partial improvement within the first 2 months (Fig. 1), with no statistical differences when comparing the OD recovery with the cases group (p = 0.95).
Regarding GD in the case group, 88% of the patients presented GD recovery, being complete in 73.6% and partial in 14.4%, while 12% of the patients do not show recovery after 6 months follow-up.
Concerning the symptoms presented in COVID patients, in the statistical analysis performed only dyspnea and nasal obstruction are predictors of poor olfactory and gustatory recovery (p < 0.05). Neither of the other variables showed any positive or negative relation regarding the evolution of OD and GD.
Discussion
To our knowledge, we report the longest follow-up case–control study (6 months) in COVID-patients with OD and/or GD to date. COVID-related OD and GD have a variable incidence in the literature reviewed [20]. In Chinese studies, prevalence is around 5 and 8% for OD and GD. In comparison, in Europe, it reaches 85 and 89% [21]. Different theories have been put forward to explain these differences. First, the divergence could be due to surface protein mutations in the virus (spike protein, SP; and nucleocapsid protein, NP) while expanding worldwide. In this case, it can affect the clinical manifestations significantly due to the crucial role in virus entrance and the transcription inside cells [13]. On the other hand, differences in ACE-2 receptors between the Asian and European population is identified as another point of discussion regarding this topic, although more studies are needed in this field. Third, in the initial COVID-19 outbreak in China physicians might have focused on severe primary symptoms and therefore OD and GD symptoms could have been missed [12].
In our study, OD and GD correspond to 64.1 and 60.5% of the positive COVID patients, respectively. Furthermore, the presence of hyposmia (OR 13.89), hypogeusia (OR 17.6), and dysosmia (OR 14.94) were strongly associated with a positive RT-PCR. These results show a higher number of patients affected by OD and GD in comparison with the metanalysis published by Tong et al. [1], which may be influenced by the cohort of patients (health workers) and a high proportion of women (81%), young (71% less than 50 years old) and healthy subjects (90% with none or one comorbidity). Previous studies are showing the relationship between this type of patient and the high incidence of OD and GD [7]. Furthermore, the high number of study participants who completed a long-term follow-up (6 months) is possible due to better access to patients as they work in the hospital.
The average time of the OD and GD onset, as the first symptoms, is 4.65 (± 4.42) days. As stated in other studies [6, 13], hyposmia is the first symptom (11.2%) or begins simultaneously with other symptoms (14.4%) in 25.6% of our patients. In the same way, the value of hyposmia in screening is interesting, according to the results of the Anosmia Reporting Tool, anosmia contributed to testing for COVID-19 in 40% of the patients [6].
An important point to analyze is fever concerning hyposmia in COVID screening. Current COVID-19 screening protocols are usually oriented towards the determination of fever, but our results show, that only 34% of them suffer from it. Due to the high prevalence of OD in the early stages of the disease, hyposmia could be an optimal indicator for COVID screening [22]. In the same way, the knowledge of this symptom by the general population could be useful for the development of strategies to minimize the risk of contagion, for example by using self-tests of OD, self-isolation, etc. [23]. The design of objective tests for hyposmia screening could also help, as other studies highlight there is a significant percentage of patients who subjectively deny hyposmia, but this dysfunction is demonstrated in an objective evaluation [24].
Either OD or GD in COVID shows a low correlation with other nasal symptoms like nasal obstruction, suggesting a primary chemosensory dysfunction, and not a mere obstruction of airflow to the olfactory reception area. This may be due to a high expression of ACE in the nasal mucosa, as previously state, the high neurotropism of the coronavirus [12]. In our study, nasal obstruction is only associated with 23.4% of patients with OD. The absence of nasal obstruction in patients with OD may be useful for the differential diagnosis of hyposmia with other upper respiratory infections.
In our investigation, we did not find a relationship between the clinical history and the development of OD and GD symptoms in either group. The mean OD recovery time in the case-group is 30.37 days (± 28.11 days), while in the control group it is 23.27 days (± 18.2 days). In both groups, within the first 8 weeks, many patients have a complete or partial recovery, 83.2% in the case group, 100% in the control group. However, the recovery curves of the case group patients who did not recover in that time show a plateau pattern, with a flattening starting 2 months after the onset of symptoms. At the end of the study, at 6 months of follow-up; the recovery rate in the case group is 88.8%, while 14 patients (11.2%) still do not show recovery. It should be considered that within improvement cases, 30% of the subjects do not completely recover their previous sense of smell.
A significant result of our study, not published before as far as we know, is that dyspnea (p = 0.034) is identified as a poor predictor regarding OD recovery in the case group. On the other hand, as published by Paderno et al., nasal obstruction (p = 0.02) is a predictor for non-recovery OD/GD symptoms [10]. Other studies on this topic would be interesting to support our results.
In the same way, there is no correlation between OD/GD recovery and a negative PCR [10]. In our case, only 53% of the patients have improved then. It seems clear that in certain patients, OD/GD may persist even after the infection is resolved. Besides, recent publications state that there is no relationship between viral load and the development of GD/OD symptoms [25]. Similarly, in our study, the development of antibodies after the infection does not represent any predictive factor for the development of OD/GD, or its evolution.
Although most publications agree on the close correlation between GD and OD in COVID patients, some authors emphasize that they may represent an independent clinical entity [18]. In our work, there is a significant positive association between OD and GD in the diagnosis (r2 0.714), as well as a strong correlation of the VAS in the recovery of both symptoms (r2 0.684). This suggests that the symptoms are related. Another aspect to highlight is that 24% of the COVID patients with OD report dysosmia, with parosmia being the most frequent form presented (73%). In the same way, this is the first study to report the odds of having dysosmia after an initial positive RT-PCR compared to the control group (OR 3.86). This result should lead us to emphasize the analysis of qualitative variables of the olfactory function since it can be an overlooked symptom.
Implications for practice
Currently, there is no consensus on the management of COVID patients with OD and GD. Due to the high rate of recovery in the first weeks, some studies recommend a conservative treatment [16], while other authors proposed olfactory rehabilitation and early treatment, such as intranasal vitamin A and systemic omega-3 [26]. Residual OD/GD could be considered as a minor sequela of COVID, but this pathology seems to present a high prevalence with an impact on the quality of life of patients [27]. Health professionals must anticipate a probable increase in olfactory dysfunction in the coming months due to COVID, plan adequate management of this pathology, evaluate medical treatments, and develop olfactory rehabilitation programs [26].
Limitations
One limitation of our study is related to the targeted population size. Since they were attended in case of suspicion of a COVID-19 infection, our percentage of totally asymptomatic subjects is probably abnormally low. In the same way, the high percentage of female and relatively young patients is biased because of the patient’s status as a health worker. We should emphasize that our high percentage of the female population should prevent generalizing our results to other male populations.
The use of VAS and the lack of objective olfactory/gustatory testing is a limitation in our study due to the poor correlation between subjective reporting and objective chemosensory testing. VAS is used in this study as the only parameter because of the critical situation in the COVID-19 period. This situation could also explain the high variability published about OD recovery in common cold patients [28, 29].
Despite the limitations, the data presented here may constitute a valuable help for future prospective studies with longer follow-up periods investigating these associations.
Conclusions
OD and GD show high-resolution rates during the first two months after the onset of symptoms in COVID patients. Despite this, 11% of the patients do not present recovery, and in up to 30% the recovery is partial, at 6 months of follow-up. There is a high correlation between OD and GD, both in the appearance of symptoms and in their recovery. Nasal obstruction and dyspnea have been identified as risk factors for the persistence of symptoms.
References
Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T (2020) The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 163(1):3–11. https://doi.org/10.1177/0194599820926473
Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M et al (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26(5):681–687
Welge-Lüssen A, Wolfensberger M (2006) Olfactory disorders following upper respiratory tract infections definition of postviral olfactory disorders. Adv Otorhinolaryngol 63:125–132
Auinger AB, Besser G, Liu DT, Renner B, Mueller CA (2020) Long-term impact of olfactory dysfunction on daily life. Wien Klin Wochenschr. https://doi.org/10.1007/s00508-020-01751-5
Bilinska K, Butowt R (2020) Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem Neurosci 11(15):2152–2155
Wheeler DL, Athmer J, Meyerholz DK, Perlman S (2017) Murine olfactory bulb interneurons survive infection with a neurotropic coronavirus. J Virol 91(22):319–335
Sayin İ, Yaşar KK, Yazici ZM (2020) Taste and smell impairment in COVID-19: an AAO-HNS anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg 163(3):473–479
Martin-Sanz E, Riestra J, Yebra L, Larran A, Mancino F, Yanes-Diaz J et al (2020) Prospective study in 355 patients with suspected COVID-19 infection: value of cough, subjective hyposmia, and hypogeusia. Laryngoscope 130(11):2674–2679
Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC (2020) COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg 163(1):132–134
Lechien JR, Chiesa-Estomba CM, Hans S, Barillari MR, Jouffe L, Saussez S (2020) Loss of smell and taste in 2013 european patients with mild to moderate COVID-19. Ann Intern Med 173(8):672–675
Liu DT, Besser G, Prem B, Sharma G, Koenighofer M, Renner B et al (2020) Association between orthonasal olfaction and chemosensory perception in patients with smell loss. Laryngoscope 130(9):2213–2219
Niklassen AS, Draf J, Huart C, Hintschich C, Bocksberger S, Trecca EMC et al (2021) COVID-19: recovery from chemosensory dysfunction. A multicentre study on smell and taste. Laryngoscope. https://doi.org/10.1002/lary.29383
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X et al (2020) High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 12(1):1–5
Bigiani A (2020) Gustatory dysfunctions in COVID-19 patients: possible involvement of taste renin-angiotensin system (RAS). Eur Arch Otorhinolaryngol 277(8):2395
Lechien JR, Hsieh JW, Ayad T, Fakhry N, Hans S, Chiesa-Estomba CM et al (2020) Gustatory dysfunctions in COVID-19. Eur Arch Otorhinolaryngol 277(8):2397–2398
Sedaghat AR, Gengler I, Speth MM (2020) Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg 163(1):12–15
Paderno A, Mattavelli D, Rampinelli V, Grammatica A, Raffetti E, Tomasoni M et al (2020) Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg Otolaryngol Head Neck Surg 163(6):1144–1149
Lee Y, Min P, Lee S, Kim SW (2020) Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci 35(18):1–6
Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR (2020) Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg 163(1):114–120
Meng X, Deng Y, Dai Z, Meng Z (2020) COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol 41(5):102581
Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277(8):2251–2261. https://doi.org/10.1007/s00405-020-05965-1
Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA et al (2020) Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 26(7):1037–1040. https://doi.org/10.1038/s41591-020-0916-2
Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G (2020) Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck 42(7):1570–1576
Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M et al (2020) Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 42(7):1560–1569
Cho RHW, To ZWH, Yeung ZWC, Tso EYK, Fung KSC, Chau SKY et al (2020) COVID-19 viral load in the severity of and recovery from olfactory and gustatory dysfunction. Laryngoscope 130(11):2680–2685
Whitcroft KL, Hummel T (2020) Olfactory dysfunction in COVID-19: diagnosis and management. JAMA 323(24):2512–2514
Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L (2020) Management of post-acute covid-19 in primary care. BMJ 370:17006637
Reden J, Mueller A, Mueller C, Konstantinidis I, Frasnelli J, Landis BN, Hummel T (2006) Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg 132(3):265–269
Lee DY, Lee WH, Wee JH, Kim JW (2014) Prognosis of postviral olfactory loss: follow-up study for longer than one year. Am J Rhinol Allergy 28(5):419–422
Funding
None.
Author information
Authors and Affiliations
Contributions
JR-A: design, analysis, acquisition data, drafting the article; JY-D: design, analyze data, drafting the article; JE-S: design, interpret data, revise the article; CV: acquisition and interpretation data, revise the article; CM-Q: acquisition and interpretation data, drafting the article; AL-J: analysis data, drafting the article, revise the article; EM-S: design, analyze data, revise the article.
Corresponding author
Ethics declarations
Conflict of interest
No, no sponsorships or competing interests have been disclosed for this article.
Ethics approval
The institutional review board, CEIM University Hospital of Getafe (CV20/24), approved this project.
Research involving human participants and/or animals
Yes, this study involves human participants.
Informed consent
Yes, patient informed consent was obtained from every patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Riestra-Ayora, J., Yanes-Diaz, J., Esteban-Sanchez, J. et al. Long-term follow-up of olfactory and gustatory dysfunction in COVID-19: 6 months case–control study of health workers. Eur Arch Otorhinolaryngol 278, 4831–4837 (2021). https://doi.org/10.1007/s00405-021-06764-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06764-y