Abstract
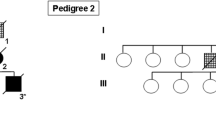
Globular glial tauopathies (GGTs) are 4-repeat tauopathies neuropathologically characterized by tau-positive, globular glial inclusions, including both globular oligodendroglial inclusions and globular astrocytic inclusions. No mutations have been found in 25 of the 30 GGT cases reported in the literature who have been screened for mutations in microtubule associated protein tau (MAPT). In this report, six patients with GGT (four with subtype III and two with subtype I) were screened for MAPT mutations. They included 4 men and 2 women with a mean age at death of 73 years (55–83 years) and mean age at symptomatic onset of 66 years (50–77 years). Disease duration ranged from 5 to 14 years. All were homozygous for the MAPT H1 haplotype. Three patients had a positive family history of dementia, and a novel MAPT mutation (c.951G>C, p.K317N) was identified in one of them, a patient with subtype III. Recombinant tau protein bearing the lysine-to-asparagine substitution at amino acid residue 317 was used to assess functional significance of the variant on microtubule assembly and tau filament formation. Recombinant p.K317N tau had reduced ability to promote tubulin polymerization. Recombinant 3R and 4R tau bearing the p.K317N mutation showed decreased 3R tau and increased 4R tau filament assembly. These results strongly suggest that the p.K317N variant is pathogenic. Sequencing of MAPT should be considered in patients with GGT and a family history of dementia or movement disorder. Since several individuals in our series had a positive family history but no MAPT mutation, genetic factors other than MAPT may play a role in disease pathogenesis.







Similar content being viewed by others
References
Adams SJ, DeTure MA, McBride M, Dickson DW, Petrucelli L (2010) Three repeat isoforms of tau inhibit assembly of four repeat tau filaments. PLoS One 5:e10810
Ahmed Z, Bigio EH, Budka H et al (2013) Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol 126:537–544
Ahmed Z, Doherty KM, Silveira-Moriyama L et al (2011) Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol 122:415–428
Alafuzoff I, Pikkarainen M, Al-Sarraj S et al (2006) Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol 65:740–757
Andreadis A, Brown WM, Kosik KS (1992) Structure and novel exons of the human tau gene. Biochemistry 31:10626–10633
Arai T, Ikeda K, Akiyama H et al (2004) Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol 55:72–79
Bigio EH, Lipton AM, Yen SH et al (2001) Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol 60:328–341
Braak H, Braak E (1987) Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci Lett 76:124–127
Brandt R, Hundelt M, Shahani N (2005) Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta 1739:331–354
Buee L, Delacourte A (1999) Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol 9:681–693
Cook C, Carlomagno Y, Gendron TF et al (2014) Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum Mol Genet 23:104–116
DeTure M, Ko LW, Yen S et al (2000) Missense tau mutations identified in FTDP-17 have a small effect on tau–microtubule interactions. Brain Res 853:5–14
Duyckaerts C, Delaere P, Hauw JJ et al (1990) Rating of the lesions in senile dementia of the Alzheimer type: concordance between laboratories. A European multicenter study under the auspices of EURAGE. J Neurol Sci 97:295–323
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Ferrer I, Hernandez I, Boada M et al (2003) Primary progressive aphasia as the initial manifestation of corticobasal degeneration and unusual tauopathies. Acta Neuropathol 106:419–435
Ferrer I, Lopez-Gonzalez I, Carmona M et al (2014) Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol 73:81–97
Fu YJ, Nishihira Y, Kuroda S et al (2010) Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: a distinct clinicopathological and biochemical disease entity. Acta Neuropathol 120:21–32
Gelpi E, Cullel F, Navarro-Otano J, Llado A (2013) Globular glial-like inclusions in a patient with advanced Alzheimer’s disease. Acta Neuropathol 126:155–157
Ghetti B, Wszolek ZK, Boeve BF, Spina S, Goedert M (2011) Frontotemporal dementia and parkinsonism linked to chromosome 17. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Wiley-Blackwell, West Sussex, pp 110–134
Giaccone G, Marcon G, Mangieri M et al (2008) Atypical tauopathy with massive involvement of the white matter. Neuropathol Appl Neurobiol 34:468–472
Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA (1989) Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 8:393–399
Hayashi S, Toyoshima Y, Hasegawa M et al (2002) Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann Neurol 51:525–530
Josephs KA, Dickson DW (2003) Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord 18:1018–1026
Josephs KA, Duffy JR, Strand EA et al (2012) Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 135:1522–1536
Josephs KA, Katsuse O, Beccano-Kelly DA et al (2006) Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol 65:396–405
Josephs KA, Murray ME, Whitwell JL et al (2014) Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 127:441–450
Komori T, Arai N, Oda M et al (1998) Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 96:401–408
Kouri N, Carlomagno Y, Baker M et al (2014) Novel mutation in MAPT exon 13 (p. N410H) causes corticobasal degeneration. Acta Neuropathol 127:271–282
Kouri N, Murray ME, Hassan A et al (2011) Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain 134:3264–3275
Kovacs GG, Majtenyi K, Spina S et al (2008) White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67:963–975
Kovacs GG, Milenkovic I, Wohrer A et al (2013) Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 126:365–384
Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159
Maeda S, Sahara N, Saito Y et al (2007) Granular tau oligomers as intermediates of tau filaments. Biochemistry 46:3856–3861
Mandelkow E, Thies E, Trinczek B, Biernat J, Mandelkow E (2004) MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol 167:99–110
Mirra SS, Gearing M, McKeel DW Jr et al (1994) Interlaboratory comparison of neuropathology assessments in Alzheimer’s disease: a study of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). J Neuropathol Exp Neurol 53:303–315
Molina JA, Probst A, Villanueva C et al (1998) Primary progressive aphasia with glial cytoplasmic inclusions. Eur Neurol 40:71–77
Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA (1986) Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res 387:271–280
Piao YS, Tan CF, Iwanaga K et al (2005) Sporadic four-repeat tauopathy with frontotemporal degeneration, parkinsonism and motor neuron disease. Acta Neuropathol 110:600–609
Powers JM, Byrne NP, Ito M et al (2003) A novel leukoencephalopathy associated with tau deposits primarily in white matter glia. Acta Neuropathol 106:181–187
Rademakers R, Cruts M, van Broeckhoven C (2004) The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 24:277–295
Rosso SM, van Herpen E, Deelen W et al (2002) A novel tau mutation, S320F, causes a tauopathy with inclusions similar to those in Pick’s disease. Ann Neurol 51:373–376
Sahara N, Lewis J, DeTure M et al (2002) Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J Neurochem 83:1498–1508
Santa-Maria I, Perez M, Hernandez F, Avila J, Moreno FJ (2006) Characteristics of the binding of thioflavin S to tau paired helical filaments. J Alzheimer’s Dis: JAD 9:279–285
Togo T, Cookson N, Dickson DW (2002) Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol 12:45–52
Togo T, Sahara N, Yen SH et al (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556
Tolnay M, Schwietert M, Monsch AU, Staehelin HB, Langui D, Probst A (1997) Argyrophilic grain disease: distribution of grains in patients with and without dementia. Acta Neuropathol 94:353–358
Uchihara T (2007) Silver diagnosis in neuropathology: principles, practice and revised interpretation. Acta Neuropathol 113:483–499
Uchikado H, Lin WL, DeLucia MW, Dickson DW (2006) Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 65:685–697
van Herpen E, Rosso SM, Serverijnen LA et al (2003) Variable phenotypic expression and extensive tau pathology in two families with the novel tau mutation L315R. Ann Neurol 54:573–581
Yoshida M (2006) Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology 26:457–470
Zarranz JJ, Ferrer I, Lezcano E et al (2005) A novel mutation (K317M) in the MAPT gene causes FTDP and motor neuron disease. Neurology 64:1578–1585
Acknowledgments
This study was supported by the NIH P50 NS072187 (DWD, ZKW, OAR, RR, AJS), the Max Kade Foundation (PT), NIH R01 NS078086 (OAR), NIH R01 NS080882 (RR), NIH R01 NS065782 (RR), NIH R01 AG026251 (RR), NIH P50 AG16574 (RR), the ALS Therapy Alliance (RR), the Consortium for Frontotemporal Degeneration Research (RR), the NIH R01 DC010367 (KAJ), the NIH R01 AG037491 (KAJ), the NIH R01 DC012519 (KAJ), and the Alzheimer’s Association (KAJ), the Mayo Clinic Foundation (LP), the NIH P50 AG016574 (LP), the NIH R01 NS089544-1 (LP), the BrightFocus Foundation (LP), the gift from Carl Edward Bolch, Jr., and Susan Bass Bolch Foundation (SF, ZKW). We are grateful to all patients, family members, and caregivers who agreed to brain donation; without their donation these studies would have been impossible. We also acknowledge expert technical assistance of Linda Rousseau and Virginia Phillips for histology and Monica Castanedes-Casey for immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Tacik and M. DeTure have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2015_1425_MOESM1_ESM.tif
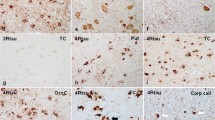
Supplementary material 1 (TIFF 7578 kb) Fig. 1 Pathologic characterization of 6 GGT cases with of phospho-tau (CP13) and 4R tau (RD4) tau immunohistochemistry as well as Gallyas sliver stain of the most severely affected areas of the white and gray matter, including motor cortex (case 1, subtype III; case 5, subtype III; case 6, subtype III), temporal lobe (case 2, subtype I; case 4, subtype I), and superior frontal gyrus (case 3, subtype III). Scale bar = 20 µm
401_2015_1425_MOESM2_ESM.tif
Supplementary material 2 (TIFF 4841 kb) Fig. 2 Chromatogram of novel MAPT p.K317N mutation. The arrow indicates the mutation site within exon 11 of MAPT
401_2015_1425_MOESM3_ESM.tif
Supplementary material 3 (TIFF 464 kb) Fig 3 (a) Effects of 4R0N p.K317N on aggregation. Tau aggregation induced by incubation of recombinant tau with dextran sulfate. Aggregation is 10% greater in 4R0N p.K317N (gray bars) than 4R0N (black bars) at 2 hours, but not different at 20 hours. (*p < 0.01); (b) Effects of 4R0N p.K317N on misfolding assessed by thioflavin S fluorescence. Tau misfolding is less in 4R0N p.K317N (gray bars), compared to 4R0N (black bars) at both 2 and 20 hours. (*p < 0.05)
401_2015_1425_MOESM4_ESM.tif
Supplementary material 4 (TIFF 23686 kb) Fig 4 Electron micrographs of recombinant 4R0N filaments (4R WT) and 4R0N p.K317N (4R K317N) at 1 hour. The scale bar = 200 nm
Rights and permissions
About this article
Cite this article
Tacik, P., DeTure, M., Lin, WL. et al. A novel tau mutation, p.K317N, causes globular glial tauopathy. Acta Neuropathol 130, 199–214 (2015). https://doi.org/10.1007/s00401-015-1425-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1425-0