Abstract
Purpose
Submucosal collagen is paramount for colonic anastomotic integrity. Matrix metalloproteinases (MMPs) mediate collagen degradation that increases the risk of wound dehiscence. Although broad-spectrum MMP inhibitors are beneficial for anastomotic strength, they can cause adverse reactions. Knowledge of specific MMPs responsible for the weakening of anastomoses can be used to optimise MMP inhibition therapy. We aimed to quantify transcript and protein levels of multiple MMPs in colonic anastomoses and evaluate the effect of inhibiting the MMPs that displayed the highest expression levels on anastomotic repair.
Methods
Left-sided colonic anastomoses were made in male Sprague-Dawley rats. After 3 days when biomechanical strength is lowest, MMP mRNA and protein levels were measured by quantitative real-time polymerase chain reaction, enzyme-linked immunosorbent assays and gelatin zymography. The effects of the MMP-8, MMP-9 and MMP-12 synthetic inhibitor AZD3342 was also studied.
Results
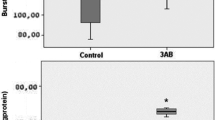
MMP-8, MMP-9 and MMP-12 gene and protein expression increased profoundly (p < 0.01), and MMP-13 mRNA and MMP-2 mRNA and protein modestly (p < 0.001) in the anastomoses. MMP-3 mRNA levels were not up-regulated significantly compared with adjacent uninjured colon. Increased anastomotic MMP-12 levels paralleled macrophage infiltration by immunohistochemical analyses. AZD3342 (50 mg/kg) treatment increased the anastomotic breaking strength by 29 % (p = 0.015) day 3 compared with vehicle. Improved anastomotic strength was not accompanied with alterations of type I or type III procollagen mRNA but was possibly due to inhibition of the concerted digestive action on the existent submucosal collagens by the targeted MMPs.
Conclusion
The present findings justify the concept of selective MMP inhibition to enhance anastomotic strength in colon.




Similar content being viewed by others
References
Khan AA, Wheeler JM, Cunningham C, George B, Kettlewell M, Mortensen NJ (2008) The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis 10:587–592
Kube R, Mroczkowski P, Granowski D, Benedix F, Sahm M, Schmidt U et al (2010) Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur J Surg Oncol 36:120–124
Morrison CJ, Butler GS, Rodriguez D, Overall CM (2009) Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol 21:645–653
Halsted WS (1887) Circular suture of the intestine—an experimental study. Am J Med Sci 188:436–460
Syk I, Ågren MS, Adawi D, Jeppsson B (2001) Inhibition of matrix metalloproteinases enhances breaking strength of colonic anastomoses in an experimental model. Br J Surg 88:228–234
de Hingh IH, Siemonsma MA, de Man BM, Lomme RM, Hendriks T (2002) The matrix metalloproteinase inhibitor BB-94 improves the strength of intestinal anastomoses in the rat. Int J Colorectal Dis 17:348–354
Ågren MS, Andersen L, Heegaard AM, Jorgensen LN (2008) Effect of parenteral zinc sulfate on colon anastomosis repair in the rat. Int J Colorectal Dis 23:857–861
van der Stappen JW, Hendriks T, de Boer HH, de Man BM, de Pont JJ (1992) Collagenolytic activity in experimental intestinal anastomoses. Differences between small and large bowel and evidence for the presence of collagenase. Int J Colorectal Dis 7:95–101
Seifert WF, Wobbes T, Hendriks T (1996) Divergent patterns of matrix metalloproteinase activity during wound healing in ileum and colon of rats. Gut 39:114–119
Savage FJ, Lacombe DL, Boulos PB, Hembry RM (1997) Role of matrix metalloproteinases in healing of colonic anastomosis. Dis Colon Rectum 40:962–970
Ågren MS, Andersen TL, Mirastschijski U, Syk I, Schiødt CB, Surve V et al (2006) Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery 140:72–82
Stumpf M, Klinge U, Wilms A, Zabrocki R, Rosch R, Junge K et al (2005) Changes of the extracellular matrix as a risk factor for anastomotic leakage after large bowel surgery. Surgery 137:229–234
Pasternak B, Matthiessen P, Jansson K, Andersson M, Aspenberg P (2010) Elevated intraperitoneal matrix metalloproteinases-8 and -9 in patients who develop anastomotic leakage after rectal cancer surgery: a pilot study. Colorectal Dis 12:e93–e98
Kiyama T, Onda M, Tokunaga A, Efron DT, Barbul A (2001) Effect of matrix metalloproteinase inhibition on colonic anastomotic healing in rats. J Gastrointest Surg 5:303–311
Ågren MS, Andersen TL, Andersen L, Schiødt CB, Surve V, Andreassen TT et al (2011) Nonselective matrix metalloproteinase but not tumor necrosis factor-alpha inhibition effectively preserves the early critical colon anastomotic integrity. Int J Colorectal Dis 26:329–337
Renkiewicz R, Qiu L, Lesch C, Sun X, Devalaraja R, Cody T et al (2003) Broad-spectrum matrix metalloproteinase inhibitor marimastat-induced musculoskeletal side effects in rats. Arthritis Rheum 48:1742–1749
Björnsson MJ, Havemose-Poulsen A, Stoltze K, Holmstrup P (2004) Influence of the matrix metalloproteinase inhibitor batimastat (BB-94) on periodontal bone destruction in Sprague–Dawley rats. J Periodontal Res 39:269–274
Hutchinson JW, Tierney GM, Parsons SL, Davis TR (1998) Dupuytren’s disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. J Bone Joint Surg Br 80:907–908
Drummond AH, Beckett P, Brown PD, Bone EA, Davidson AH, Galloway WA et al (1999) Preclinical and clinical studies of MMP inhibitors in cancer. Ann N Y Acad Sci 878:228–235
Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U (1998) Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol 152:1005–1014
Madlener M, Parks WC, Werner S (1998) Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res 242:201–210
Gawronska-Kozak B (2011) Scarless skin wound healing in FOXN1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biol 30:290–300
Shapiro SD, Kobayashi DK, Pentland AP, Welgus HG (1993) Induction of macrophage metalloproteinases by extracellular matrix. Evidence for enzyme- and substrate-specific responses involving prostaglandin-dependent mechanisms. J Biol Chem 268:8170–8175
Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD (1996) Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A 93:3942–3946
Taddese S, Jung MC, Ihling C, Heinz A, Neubert RH, Schmelzer CE (2010) MMP-12 catalytic domain recognizes and cleaves at multiple sites in human skin collagen type I and type III. Biochim Biophys Acta 1804:731–739
Danielsen PL, Holst AV, Maltesen HR, Bassi MR, Holst PJ, Heinemeier KM et al (2011) Matrix metalloproteinase-8 overexpression prevents proper tissue repair. Surgery 150:897–906
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162:156–159
Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A (2004) Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 37:112–114, 116, 118–119
Mirastschijski U, Impola U, Karsdal MA, Saarialho-Kere U, Ågren MS (2002) Matrix metalloproteinase inhibitor BB-3103 unlike the serine proteinase inhibitor aprotinin abrogates epidermal healing of human skin wounds ex vivo. J Invest Dermatol 118:55–64
Hu X, Beeton C (2010) Detection of functional matrix metalloproteinases by zymography. J Vis Exp doi: 10.3791/2445
Carter CA, Misra M (2010) Effects of short-term cigarette smoke exposure on Fischer 344 rats and on selected lung proteins. Toxicol Pathol 38:402–415
Peeters-Joris C, Hammani K, Singer CF (1998) Differential regulation of MMP-13 (collagenase-3) and MMP-3 (stromelysin-1) in mouse calvariae. Biochim Biophys Acta 1405:14–28
Gill SE, Parks WC (2008) Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 40:1334–1347
Mirastschijski U, Haaksma CJ, Tomasek JJ, Ågren MS (2004) Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res 299:465–475
Verhofstad MH, Lange WP, van der Laak JA, Verhofstad AA, Hendriks T (2001) Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum 44:423–431
Varani J, Perone P, Fligiel SE, Fisher GJ, Voorhees JJ (2002) Inhibition of type I procollagen production in photodamage: correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J Invest Dermatol 119:122–129
Bannikov GA, Karelina TV, Collier IE, Marmer BL, Goldberg GI (2002) Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J Biol Chem 277:16022–16027
Mäkitalo L, Kolho KL, Karikoski R, Anthoni H, Saarialho-Kere U (2010) Expression profiles of matrix metalloproteinases and their inhibitors in colonic inflammation related to pediatric inflammatory bowel disease. Scand J Gastroenterol 45:862–871
Wright RW, Allen T, El-Zawawy HB, Brodt MD, Silva MJ, Gill CS et al (2006) Medial collateral ligament healing in macrophage metalloelastase (MMP-12)-deficient mice. J Orthop Res 24:2106–2113
Houghton AM, Hartzell WO, Robbins CS, Gomis-Ruth FX, Shapiro SD (2009) Macrophage elastase kills bacteria within murine macrophages. Nature 460:637–641
Witte MB, Thornton FJ, Kiyama T, Efron DT, Schulz GS, Moldawer LL et al (1998) Metalloproteinase inhibitors and wound healing: a novel enhancer of wound strength. Surgery 124:464–470
Acknowledgments
We thank Peter Schjerling for his advice on the mRNA measurements and Karen Bonde Larsen for help with microphotography. All materials and utilities were funded by the I.M. Dæhnfeldt Foundation, The A.P. Møller Foundation, The Danish Medical Association and AstraZeneca, Sweden. The authors have no disclosers to declare, except M. B. H. who is currently employed by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krarup, PM., Eld, M., Heinemeier, K. et al. Expression and inhibition of matrix metalloproteinase (MMP)-8, MMP-9 and MMP-12 in early colonic anastomotic repair. Int J Colorectal Dis 28, 1151–1159 (2013). https://doi.org/10.1007/s00384-013-1697-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-013-1697-6