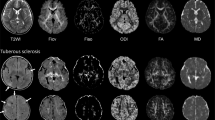
Abstract
Introduction
The contribution of radial migration lines (RMLs) to the neuroanatomical and neurocognitive phenotype of tuberous sclerosis complex (TSC) is unclear. The aim of this study was to perform a comprehensive evaluation of the neuroradiological phenotype of TSC, distinguishing RMLs from normal-appearing white matter (NAWM) using diffusion tensor imaging (DTI) and volumetric fluid-attenuated inversion recovery imaging.
Methods
Magnetic resonance images of 30 patients with TSC were evaluated. The frequencies of RMLs, tubers, and subependymal nodules (SENs) were determined for every hemispheric lobe. Cerebellar lesions and subependymal giant cell tumors were counted. DTI metrics were obtained from the NAWM of every hemispheric lobe and from the largest RML and tuber. Analyses of variance and correlations were performed to investigate the associations between neuroanatomical characteristics and relationships between RML frequency and neurocognitive outcomes. NAWM DTI metrics were compared with measurements of 16 control patients.
Results
A mean of 47 RMLs, 27 tubers, and 10 SENs were found per patient, and the frequencies of these lesions were strongly correlated (p < 0.001). RML fractional anisotropy and mean diffusivity were strongly inversely correlated (p = 0.003). NAWM DTI metrics were similar to the controls (p = 0.26). RML frequency was strongly associated with age of seizure onset (p = 0.003), intelligence outcomes (p = 0.01), and level of autistic features (p = 0.007).
Conclusion
A detailed neuroradiological phenotype is presented, showing that RMLs are the most frequent neuroanatomical lesion, are responsible for white matter DTI abnormalities, and are strongly associated with age of seizure onset, intelligence outcomes, and level of autistic features.



Similar content being viewed by others
References
Roach ES, Sparagana SP (2004) Diagnosis of tuberous sclerosis complex. J Child Neurol 19:643–649
Ridler K, Bullmore ET, De Vries PJ, Suckling J, Barker GJ, Meara SJ, Williams SC, Bolton PF (2001) Widespread anatomical abnormalities of grey and white matter structure in tuberous sclerosis. Psychol Med 31:1437–1446
DiMario FJ Jr (2004) Brain abnormalities in tuberous sclerosis complex. J Child Neurol 19:650–657
Muzykewicz DA, Newberry P, Danforth N, Halpern EF, Thiele EA (2007) Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy Behav 11:506–513
Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA (2010) The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 51:1236–1241
Jansen FE, Vincken KL, Algra A, Anbeek P, Braams O, Nellist M, Zonnenberg BA, Jennekens-Schinkel A, van den Ouweland A, Halley D, van Huffelen AC, van Nieuwenhuizen O (2008) Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology 70:916–923
Wong V, Khong PL (2006) Tuberous sclerosis complex: correlation of magnetic resonance imaging (MRI) findings with comorbidities. J Child Neurol 21:99–105
Nie D, Di Nardo A, Han JM, Baharanyi H, Kramvis I, Huynh T, Dabora S, Codeluppi S, Pandolfi PP, Pasquale EB, Sahin M (2010) Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci 13:163–172
Garaci FG, Floris R, Bozzao A, Manenti G, Simonetti A, Lupattelli T, Curatolo P, Simonetti G (2004) Increased brain apparent diffusion coefficient in tuberous sclerosis. Radiology 232:461–465
Karadag D, Mentzel HJ, Gullmar D, Rating T, Lobel U, Brandl U, Reichenbach JR, Kaiser WA (2005) Diffusion tensor imaging in children and adolescents with tuberous sclerosis. Pediatr Radiol 35:980–983
Peng SS, Lee WT, Wang YH, Huang KM (2004) Cerebral diffusion tensor images in children with tuberous sclerosis: a preliminary report. Pediatr Radiol 34:387–392
Piao C, Yu A, Li K, Wang Y, Qin W, Xue S (2009) Cerebral diffusion tensor imaging in tuberous sclerosis. Eur J Radiol 71:249–252
Firat AK, Karakas HM, Erdem G, Yakinci C, Bicak U (2006) Diffusion weighted MR findings of brain involvement in tuberous sclerosis. Diagn Interv Radiol 12:57–60
Sener RN (2002) Tuberous sclerosis: diffusion MRI findings in the brain. Eur Radiol 12:138–145
Boer K, Troost D, Jansen F, Nellist M, van den Ouweland AM, Geurts JJ, Spliet WG, Crino P, Aronica E (2008) Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 28:577–590
Griffiths PD, Hoggard N (2009) Distribution and conspicuity of intracranial abnormalities on MR imaging in adults with tuberous sclerosis complex: a comparison of sequences including ultrafast T2-weighted images. Epilepsia 50:2605–2610
Shepherd CW, Houser OW, Gomez MR (1995) MR findings in tuberous sclerosis complex and correlation with seizure development and mental impairment. Am J Neuroradiol 16:149
Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA (2011) Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology 76:981–986
Widjaja E, Simao G, Mahmoodabadi SZ, Ochi A, Snead OC, Rutka J, Otsubo H (2010) Diffusion tensor imaging identifies changes in normal-appearing white matter within the epileptogenic zone in tuberous sclerosis complex. Epilepsy Res 89:246–253
Makki MI, Chugani DC, Janisse J, Chugani HT (2007) Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol 28:1662–1667
Arulrajah S, Ertan G, Jordan L, Tekes A, Khaykin E, Izbudak I, Huisman TA (2009) Magnetic resonance imaging and diffusion-weighted imaging of normal-appearing white matter in children and young adults with tuberous sclerosis complex. Neuroradiology 51:781–786
Krishnan ML, Commowick O, Jeste SS, Weisenfeld N, Hans A, Gregas MC, Sahin M, Warfield SK (2010) Diffusion features of white matter in tuberous sclerosis with tractography. Pediatr Neurol 42:101–106
Simao G, Raybaud C, Chuang S, Go C, Snead OC, Widjaja E (2010) Diffusion tensor imaging of commissural and projection white matter in tuberous sclerosis complex and correlation with tuber load. AJNR Am J Neuroradiol 31:1273–1277
Peters JM, Sahin M, Vogel-Farley VK, Jeste SS, Nelson CA 3rd, Gregas MC, Prabhu SP, Scherrer B, Warfield SK (2012) Loss of white matter microstructural integrity is associated with adverse neurological outcome in tuberous sclerosis complex. Acad Radiol 19:17–25
Berument SK, Rutter M, Lord C, Pickles A, Bailey A (1999) Autism screening questionnaire: diagnostic validity. Br J Psychiatry 175:444–451
Lord C, Rutter M, Le Couteur A (1994) Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685
Granader YE, Bender HA, Zemon V, Rathi S, Nass R, Macallister WS (2010) The clinical utility of the Social Responsiveness Scale and Social Communication Questionnaire in tuberous sclerosis complex. Epilepsy Behav 18:262–266
Widjaja E, Zarei Mahmoodabadi S, Otsubo H, Snead OC, Holowka S, Bells S, Raybaud C (2009) Subcortical alterations in tissue microstructure adjacent to focal cortical dysplasia: detection at diffusion-tensor MR imaging by using magnetoencephalographic dipole cluster localization. Radiology 251:206–215
Allen JS, Damasio H, Grabowski TJ (2002) Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am J Phys Anthropol 118:341–358
Marcotte L, Aronica E, Baybis M, Crino PB (2012) Cytoarchitectural alterations are widespread in cerebral cortex in tuberous sclerosis complex. Acta Neuropathol (Berl) 123:685–693
Jansen FE, Braun KP, van Nieuwenhuizen O, Huiskamp G, Vincken KL, van Huffelen AC, van der Grond J (2003) Diffusion-weighted magnetic resonance imaging and identification of the epileptogenic tuber in patients with tuberous sclerosis. Arch Neurol 60:1580–1584
Widjaja E, Blaser S, Miller E, Kassner A, Shannon P, Chuang SH, Snead OC 3rd, Raybaud CR (2007) Evaluation of subcortical white matter and deep white matter tracts in malformations of cortical development. Epilepsia 48:1460–1469
Mizuguchi M, Takashima S (2001) Neuropathology of tuberous sclerosis. Brain Dev 23:508–515
Wong M, Crino PB (2012) Tuberous sclerosis and epilepsy: role of astrocytes. Glia 23:508–515
Crino PB (2011) mTOR: a pathogenic signaling pathway in developmental brain malformations. Trends Mol Med 17:734–742
da Lee Y, Yeh TH, Emnett RJ, White CR, Gutmann DH (2010) Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev 24:2317–2329
Gallagher A, Chu-Shore CJ, Montenegro MA, Major P, Costello DJ, Lyczkowski DA, Muzykewicz D, Doherty C, Thiele EA (2009) Associations between electroencephalographic and magnetic resonance imaging findings in tuberous sclerosis complex. Epilepsy Res 87:197–202
Major P, Rakowski S, Simon MV, Cheng ML, Eskandar E, Baron J, Leeman BA, Frosch MP, Thiele EA (2009) Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia 50:147–154
Waltereit R, Welzl H, Dichgans J, Lipp HP, Schmidt WJ, Weller M (2006) Enhanced episodic-like memory and kindling epilepsy in a rat model of tuberous sclerosis. J Neurochem 96:407–413
Weiner HL, Carlson C, Ridgway EB, Zaroff CM, Miles D, LaJoie J, Devinsky O (2006) Epilepsy surgery in young children with tuberous sclerosis: results of a novel approach. Pediatrics 117:1494–1502
van Eeghen A, Pulsifer M, Merker V, Neumeyer A, van Eeghen E, Thibert R, Cole A, Leigh F, Plotkin S, Thiele E (2013) Understanding relationships between autism, intelligence, and epilepsy: a cross-disorder approach. Dev Med Child Neur 55:146–153
Acknowledgments
We are grateful for the consent of the patients and for the thorough reading of the manuscript by Susana Boronat, M.D. This study was funded by the Herscot Center for Tuberous Sclerosis Complex and NIH/NINDS P01 NS024279.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Images and adopted nomenclature of various morphological abnormalities in patients with TSC. S1A) RML terminating in tuber (blue arrow) and gyral folding disruption (blue bracket). Disruption of the gyral folding pattern was defined as local simplification of the number of gyral folds compared to normal adjacent and contralateral cortex. Note the evidence for hypocellularity in both tubers (orange arrows), previously referred to as ‘cystic’ tubers, defined as low signal iso-intense with CSF on T1 and FLAIR sequences and high signal on T2 weighted sequences. This can be distinguished from mineralization (green arrow), defined as low signal on the susceptibility (SWI) and/or T2-weighted images. Note the confluence of the RMLs in the deep white matter (blue arrow); RMLs that shared part, but not all, of their neuroanatomy were counted as two lesions. S1B) Image of a subtle isolated RML in a female patient with an IQ of 112 and no neuropsychiatric comorbidity. MRI showed 16 small RMLs, no tubers and no SENs (PDF 193 kb)
Supplementary Fig. S2
Images showing examples of selected ROIs in one patient. A) ROIs in bilateral cerebellar NAWM, B) ROIs in largest RML and contralateral NAWM, and C) ROI placement in bilateral occipital WM. Due to the severe RML burden, no NAWM could be visually detected in the right occipital lobe and these DTI measurements were excluded from the analyses (PDF 180 kb)
Supplementary Fig. S3
Proportional distribution of RMLs and tubers in the study sample, compared with normal proportional volumes of cerebral lobes. Sagittal view of schematic drawings of two brains, depicting A) proportional volumes of lobes in humans (937 Allen, J.S. 2002), and B) proportional distribution of RMLs (R) and tubers (T) of the study sample (N = 30). The proportional volume of the insular lobe is 1.7 %, and RMLs and tubers were similarly distributed (both 1 %) (PDF 31 kb)
Rights and permissions
About this article
Cite this article
van Eeghen, A.M., Terán, L.O., Johnson, J. et al. The neuroanatomical phenotype of tuberous sclerosis complex: focus on radial migration lines. Neuroradiology 55, 1007–1014 (2013). https://doi.org/10.1007/s00234-013-1184-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-013-1184-3