Abstract
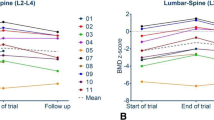
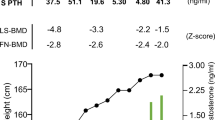
Osteogenesis imperfecta (OI) is a hereditary disease characterized by low bone mass, increased bone fragility, short stature, and skeletal deformities. This study focuses on OI type I, the mildest form of the disease. Bisphosphonates represent the prevailing standard of care in patients with OI. Teriparatide (TPD) is a PTH analog with bone-anabolic actions which has been approved for osteoporosis treatment. Thirteen postmenopausal women with type I OI who had been on treatment with neridronate for at least 2 years and who incurred new vertebral fracture during treatment were treated with TPD for 18 months. Bone mineral density (BMD) increased significantly over 18 months up to 3.5 % at the lumbar spine (p = 0.001), while no significant changes were noted in hip BMD. Serum markers of bone formation and of bone resorption increased significantly during the treatment. The Wnt inhibitors serum dickkopf-1 (DKK1) and sclerostin were also measured. A nonsignificant increase was seen in serum sclerostin levels, while serum DKK1 rose gradually and significantly during TPD treatment. In patients affected by type I OI, TPD treatment is associated with a remarkable response in markers of bone formation. This suggests a normal osteoblastic response to TPD. However, the observed increases in BMD were somewhat lower than those in postmenopausal or senile osteoporosis treated with TPD for the same lag time. Our results open the possibility to develop TPD for the treatment of adult type I OI, but particularly for the lack of a control group, a properly designed controlled study is warranted.
Similar content being viewed by others
References
Gatti D, Colapietro F, Fracassi E, Sartori E, Antoniazzi F, Braga V, Rossini M, Adami S (2003) The volumetric bone density and cortical thickness in adult patients affected by osteogenesis imperfecta. J Clin Densitom 6:173–177
Forlino A, Cabral WA, Barnes AM, Marini JC (2011) New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 7:540–557
Cheung MS, Glorieux FH (2008) Osteogenesis imperfecta: update on presentation and management. Rev Endocr Metab Disord 9:153–160
Gatti D, Antoniazzi F, Prizzi R, Braga V, Rossini M, Tatò L, Viapiana O, Adami S (2005) Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res 20:758–763
Antoniazzi F, Zamboni G, Lauriola S, Donadi L, Adami S, Tatò L (2006) Early bisphosphonate treatment in infants with severe osteogenesis imperfecta. J Pediatr 149:174–179
Adami S, Gatti D, Colapietro F, Fracassi E, Braga V, Rossini M, Tatò L (2003) Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res 18:126–130
Phillipi CA, Remmington T, Steiner RD (2008) Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev 4:CD005088
Chevrel G, Schott AM, Fontanges E, Charrin JE, Lina-Granade G, Duboeuf F, Garnero P, Arlot M, Raynal C, Meunier PJ (2006) Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebo-controlled trial. J Bone Miner Res 21:300–306
Braga V, Gatti D, Rossini M, Colapietro F, Battaglia E, Viapiana O, Adami S (2004) Bone turnover markers in patients with osteogenesis imperfecta. Bone 34:1013–1016
Girotra M, Rubin MR, Bilezikian JP (2006) The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord 7:113–121
Baron R, Rawadi G (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148:2635–2643
Ott SM (2005) Sclerostin and Wnt signaling—the pathway to bone strength. J Clin Endocrinol Metab 90:6741–6743
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot–containing protein. Am J Hum Genet 68:577–589
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875
Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39:754–766
Gatti D, Viapiana O, Idolazzi L, Fracassi E, Rossini M, Adami S (2011) The waning of teriparatide effect on bone formation markers in postmenopausal osteoporosis is associated with increasing serum levels of DKK1. J Clin Endocrinol Metab 96:1555–1559
Gatti D, Viapiana O, Adami S, Idolazzi L, Fracassi E, Rossini M (2012) Bisphosphonate treatment of postmenopausal osteoporosis is associated with a dose dependent increase in serum sclerostin. Bone 50:739–742
Gatti D, Viapiana O, Fracassi E, Idolazzi L, Dartizio C, Povino MR, Adami S, Rossini M (2012) Sclerostin and DKK1 in postmenopausal osteoporosis treated with denosumab. J Bone Miner Res 27:2259–2263
Ross AC, Taylor CL, Yaktine AL, Del Valle HB (eds) (2010) Institute of Medicine Committee to review dietary reference intakes for vitamin D and calcium dietary reference intakes for calcium and vitamin D. National Academics Press, Washington
Rossini M, Gatti D, Adami S (2013) Involvement of wnt/β-catenin signaling in the treatment of osteoporosis Calcif Tissue Int (in press)
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Boonen S, Marin F, Obermayer-Pietsch B, Simões ME, Barker C, Glass EV, Hadji P, Lyritis G, Oertel H, Nickelsen T, McCloskey EV, EUROFORS Investigators (2008) Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 93:852–860
Finkelstein JS, Wyland JJ, Lee H, Neer RM (2010) Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 95:1838–1845
Acknowledgments
We thank Caterina Fraccarollo and Cristina Bosco for the ELISAs.
Conflict of interest
S. Adami has consultant/advisory role to Amgen and Merck. All other authors have stated that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatti, D., Rossini, M., Viapiana, O. et al. Teriparatide Treatment in Adult Patients with Osteogenesis Imperfecta Type I. Calcif Tissue Int 93, 448–452 (2013). https://doi.org/10.1007/s00223-013-9770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9770-2