Abstract
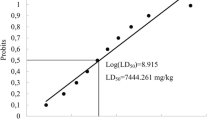
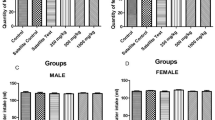
A 28-day repeated oral dose toxicity study of nonylphenol (NP) was performed for an international validation of the ‘Enhanced OECD Test Guideline 407’ paying particular attention to the sensitivity of individual endocrine-related parameters. Sprague-Dawley rats, each group consisting of ten males and ten females, were administered NP once daily by gavage at doses of 0 (control), 10, 50, or 250 mg/kg body weight. At 250 mg/kg, three females died or became moribund during the experiment. At this dose, hepatic and renal toxicity was evident in both sexes with increase of relative liver and kidney weights as well as histopathological changes, such as centrilobular liver cell hypertrophy and a variety of renal tubular lesions, and alteration of serum biochemical parameters, some of them being evident from 50 mg/kg in females (glucose and inorganic phosphates). Hematologically, development of anemia was evident at 250 mg/kg in both sexes. Regarding endocrine-related effects, increase of thyroid weight in males was detected from 50 mg/kg. At 250 mg/kg, males exhibited reduction of relative weights of the ventral prostate and seminal vesicles, and females developed irregular estrous cyclicity and vaginal mucosal hyperplasia. Although changes in serum hormone levels were detected in both sexes, magnitude of the changes was small to be regarded as a low toxicological significance. In summary, repeated oral doses of NP to rats for 28 days resulted in hepato-renal toxicity from 50 mg/kg and anemia at 250 mg/kg. Effects on the endocrine system were observed from 50 mg/kg, and assessment of weights and histopathology of endocrine-related organs and estrous cyclicity may be valid in a battery for detecting endocrine effects of NP. The no-observed-adverse-effect level of NP was estimated to be 10 mg/kg per day.


Similar content being viewed by others
References
Andrews P, Freyberger A, Hartmann E, Eiben R, Loof I, Schmidt U, Temerowski M, Folkerts A, Stahl B, Kayser M (2002) Sensitive detection of the endocrine effects of the estrogen analogue ethinylestradiol using a modified enhanced subacute rat study protocol (OECD Test Guideline no. 407). Arch Toxicol 76:194–202
Ashby J, Lefevre PA (2000) The peripubertal male rat assay as an alternative to the Hershberger castrated male rat assay for the detection of anti-androgen, oestrogens and metabolic modulators. J Appl Toxicol 20:35–47
Aso S, Anai M, Noda S, Imatanaka N, Yamasaki K, Maekawa A (2000) Twenty-eight-day repeated-dose toxicity studies for detection of weak endocrine disrupting effects of nonylphenol and atrazine in female rats. J Toxicol Pathol 13:13–20
Chapin RE, Delaney J, Wang Y, Lanning L, Davis B, Collins B, Mintz N, Wolfe G (1999) The effects of 4-nonylphenol in rats: a multigeneration reproduction study. Toxicol Sci 52:80–91
Choi SM, Lee BM (2004) An alternative mode of action of endocrine-disrupting chemicals and chemoprevention. J Toxicol Environ Health B Crit Rev 7:451–463
Cooke PS, Young P, Hess RA, Cunha GR (1991) Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology 12:2874–2879
Cunny HC, Mayes BA, Rosica KA, Trutter JA, Van Miller JP (1997) Subchronic toxicity (90-day) study with para-nonylphenol in rats. Regul Toxicol Pharmacol 26:172–178
Daston GP, Cook JC, Kavlock RJ (2003) Uncertainties for endocrine disrupters: our view on progress. Toxicol Sci 74:245–252
Fisher JS (2004) Are all EDC effects mediated via steroid hormone receptors? Toxicology 205:33–41
Green T, Swain C, Van Miller JP, Joiner RL (2003) Absorption, bioavailability, and metabolism of para-nonylphenol in the rat. Regul Toxicol Pharmacol 38:43–51
Han XD, Tu ZG, Gong Y, Shen SN, Wang XY, Kang LN, Hou YY, Chen JX (2004) The toxic effects of nonylphenol on the reproductive system of male rats. Reprod Toxicol 19:215–221
Jobling S, Reynolds T, White R, Parker MG, Sumpter J (1995) A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect 102:582–590
Kim HS, Shin JH, Moon HJ, Kang IH, Kim TS, Kim IY, Seok JH, Pyo MY, Han SY (2002) Comparative estrogenic effects of p-nonylphenol by 3-day uterotrophic assay and female pubertal onset assay. Reprod Toxicol 16:259–268
Latendresse JR, Newbold RR, Weis CC, Delclos KB (2001) Polycystic kidney disease induced in F1 Sprague-Dawley rats fed para-nonylphenol in a soy-free, casein-containing diet. Toxicol Sci 62:140–147
Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL (2000) Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci 54:154–167
Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K (2003) Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci 75:40–46
Lee PC (1998) Disruption of male reproductive tract development by administration of the xenoestrogen, nonylphenol, to male newborn rats. Endocrine 9:105–111
Nagao T, Wada K, Marumo H, Yoshimura S, Ono H (2001) Reproductive effects of nonylphenol in rats after gavage administration: a two-generation study. Reprod Toxicol 15:293–315
O’Connor JC, Frame SR, Biegel LB, Cook JC, Davis LG (1998) Sensitivity of a Tier I screening battery compared to an in utero exposure for detecting the estrogen receptor agonist 17β-estradiol. Toxicol Sci 44:169–184
Odum J, Ashby J (2000) Neonatal exposure of male rats to nonylphenol has no effect on the reproductive tract. Toxicol Sci 56:400–404
OECD (1999) OECD protocol for investigating the efficacy of the Enhanced Test Guideline 407. Organisation for Economic Co-operation and Development, Paris (issued 4 August 1999)
OECD (2000) Protocol for investigating the efficacy of the Enhanced TG 407 Test Guideline (phase 2). Rationale for the investigation, and description of the protocol. Organisation for Economic Co-operation and Development, Paris (issued 4 May 2000)
Okazaki K, Okazaki S, Nishimura S, Nakamura H, Kitamura Y, Hatayama K, Nakamura A, Tsuda T, Katsumata T, Nishikawa A, Hirose M (2001) A repeated 28-day oral dose toxicity study of methoxychlor in rats, based on the ‘Enhanced OECD Test Guideline 407’ for screening endocrine-disrupting chemicals. Arch Toxicol 75:513–521
Okazaki K, Imazawa T, Nakamura H, Furukawa F, Nishikawa A, Hirose M (2002a) A repeated 28-day oral dose toxicity study of 17α-methyltestosterone in rats, based on the ‘Enhanced OECD Test Guideline 407’ for screening endocrine-disrupting chemicals. Arch Toxicol 75:635–642
Okazaki K, Okazaki S, Nakamura H, Kitamura Y, Hatayama K, Wakabayashi S, Tsuda T, Katsumata T, Nishikawa A, Hirose M (2002b) A repeated 28-day oral dose toxicity study of genistein in rats, based on the ‘Enhanced OECD Test Guideline 407’ for screening endocrine-disrupting chemicals. Arch Toxicol 76:553–559
Okazaki S, Enami T, Nakamura H, Hatayama K, Tamura K, Numata H, Katsumata T (1998) Twenty-eight-day repeat dose oral toxicity test of nonylphenol in rats. http://wwwdb.mhlw.go.jp/ginc/dbfile1/paper/paper25154–52-3b.html
Reel JR, Lamb JC, Nea BH (1996) Survey and assessment of mammalian estrogen biological assays for hazard characterization. Fundam Appl Toxicol 34:288–305
Saunders PT, Maguire SM, Gaughan J, Millar MR (1997) Expression of oestrogen receptor β (ERβ) in multiple rat tissues visualised by immunohistochemistry. J Endocrinol 154:R13–R16
Schmutzler C, Hamann I, Hofmann PJ, Kovacs G, Stemmler L, Mentrup B, Schomburg L, Ambrugger P, Gruters A, Seidlova-Wuttke D, Jarry H, Wuttke W, Kohrle J (2004) Endocrine active compounds affect thyrotropin and thyroid hormone levels in serum as well as endpoints of thyroid hormone action in liver, heart and kidney. Toxicology 205:95–102
Schwaiger J, Spieser OH, Bauer C, Ferling H, Mallow U, Kalbfus W, Negele RD (2000) Chronic toxicity of nonylphenol and ethinylestradiol: haematological and histopathological effects in juvenile Common carp (Cyprinus carpio). Aquat Toxicol 51:69–78
Sohoni P, Sumpter JP (1998) Several environmental oestrogens are also anti-androgens. J Endocrinol 158:327–339
Takeyoshi M, Mitoma H, Yamasaki K (2005) Changes in serum α2u-globulin levels in castrated male rats treated with testosterone propionate in a Hershberger assay. J Appl Toxicol 25:176–178
Toyoda K, Shibutani M, Tamura T, Koujitani T, Uneyama C, Hirose M (2000) Repeated dose (28 days) oral toxicity study of flutamide in rats, based on the draft protocol for the ‘Enhanced OECD Test Guideline 407’ for screening for endocrine-disrupting chemicals. Arch Toxicol 74:127–132
Tyl RW, Myers CB, Marr MC, Castillo NP, Seely JC, Sloan CS, Veselica MM, Joiner RL, Van Miller JP, Simon GS (2006) Three-generation evaluation of dietary para-nonylphenol in CD® (SD) rats. Toxicol Sci 92:295–310
White R, Jobling S, Hoare SA, Sumpter JP, Parker MG (1994) Environmental persistent alkyphenolic compounds are estrogenic. Endocrinology 135:175–182
Yamasaki K, Sawaki M, Noda S, Imatanaka N, Takatsuki M (2002) Subacute oral dose toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the Enhanced OECD Test Guideline no. 407. Arch Toxicol 76:65–74
Yamasaki K, Takeyoshi M., Sawaki M, Imatanaka N, Shinoda K, Takatsuki M (2003) Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology 183:93–115
Acknowledgments
This work was supported by Health and Labour Sciences Research Grants (Risk Analysis Research on Food and Pharmaceuticals) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woo, GH., Shibutani, M., Ichiki, T. et al. A repeated 28-day oral dose toxicity study of nonylphenol in rats, based on the ‘Enhanced OECD Test Guideline 407’ for screening of endocrine-disrupting chemicals. Arch Toxicol 81, 77–88 (2007). https://doi.org/10.1007/s00204-006-0129-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-006-0129-6