Abstract
Summary
This study estimated the incidence of osteonecrosis in a Swedish, nationwide cohort of older adults. Osteonecrosis was approximately 10 times more common than in previous studies. The strongest risk factors were dialysis, hip fracture, osteomyelitis, and organ transplantation, but only hip fractures could have contributed substantially to the disease burden.
Introduction
The aim of this study was to estimate the incidence of osteonecrosis in a Swedish, nationwide cohort of older adults and in a large number of risk groups in that cohort.
Methods
In this retrospective cohort study, we included everyone who was aged 50 years or older and who was living in Sweden on 31 December 2005. We used Swedish national databases to collect data about prescription medication use, diagnosed medical conditions, and performed medical and surgical procedures. The study outcome was diagnosis of primary or secondary osteonecrosis at any skeletal site. The strength of risk factors was assessed using age- and sex-standardized incidence ratios (SIRs).
Results
The study cohort comprised 3,338,463 adults. The 10-year risk of osteonecrosis was 0.4% (n = 13,425), and the incidence rate was 4.7 cases/10000 person-years (95% confidence interval [CI], 4.6 to 4.7 cases). The strongest risk factors for osteonecrosis were hip fracture (SIR, 7.98; 95% CI, 7.69–8.27), solid organ transplantation (SIR, 7.14; 95% CI, 5.59–8.99), dialysis (SIR, 6.65; 95% CI, 5.62–7.81), and osteomyelitis (SIR, 6.43; 95% CI, 5.70–7.23). A history of hip fracture was present in 21.7% of cases of osteonecrosis, but osteomyelitis, dialysis, and solid organ transplantation were present in only 0.5 to 2% of cases.
Conclusions
Osteonecrosis was approximately 10 times more common than a small number of previous population-based studies have suggested. The strongest risk factors for osteonecrosis were dialysis, hip fracture, osteomyelitis, and solid organ transplantation, but only hip fractures could have contributed substantially to the disease burden.
Similar content being viewed by others
Introduction
Osteonecrosis (or avascular necrosis) is a potentially painful and disabling bone disease that involves the death of bone tissue [1, 2]. Risk factors for osteonecrosis include fractures [3,4,5], radiation [6, 7], corticosteroids [8, 9], excess alcohol consumption [10], and a large number of medical conditions [1, 2, 11,12,13]. The incidence of osteonecrosis in general populations is uncertain because there have been few large, population-based studies and because these few studies primarily estimated the prevalence of osteonecrosis and the incidence of particular types of osteonecrosis [14,15,16,17,18,19]. The incidence has not been comprehensively assessed in risk groups, as previous studies considered only one risk group or a limited number of risk factors [5,6,7, 14, 15, 20,21,22,23,24].
A caveat when estimating the incidence of a disease in risk groups is that any excess risk may be due to underlying characteristics instead of the risk factor of interest. Indeed, the causality of many of the medical conditions associated with osteonecrosis is unclear, as is the true effect of risk factors that are generally accepted as causal, such as corticosteroids [11, 12]. The aim of the present study was to determine how common osteonecrosis is in a general population and to identify high-risk groups, irrespective of the underlying cause of the high risk. To do so, we estimated the incidence of osteonecrosis in a Swedish, nationwide cohort of older adults and in a large number of risk groups in that cohort.
Methods
Data collection and study cohort
This study, a retrospective cohort study, was based on data collected from Swedish national databases, managed by Statistics Sweden or the Swedish National Board of Health and Welfare. We identified a study cohort using the Register of the Total Population [25]. This cohort comprised everyone aged 50 years or older who was living in Sweden on 31 December 2005.
Study outcome
The study outcome was diagnosis of primary (idiopathic) or secondary osteonecrosis, made either during hospitalization or at a visit to a specialist physician. This outcome included the following International Classification of Diseases, 10th Revision, (ICD-10) codes: M87 (osteonecrosis), M90.3 (osteonecrosis in Caisson disease), M90.4 (osteonecrosis due to hemoglobinopathy), M90.5 (osteonecrosis in other diseases classified elsewhere), M93.1 (Kienböck disease of adults), and M93.2 (osteochondritis dissecans). Although osteochondritis dissecans occurs mainly in children, we included this diagnosis in this study of adults for completeness [2]. Code K10.2 (osteonecrosis of the jaw) was not included because it also covers conditions other than osteonecrosis.
We tracked the study outcome using the National Patient Register, a database that records diagnoses made in any inpatient care in Sweden since 1987 and any specialist outpatient care since 2001 [26]. Diagnoses have been coded according to the ICD-10 since 1997, and we did not have access to prevalence data about osteonecrosis prior to 1997.
Validation of study outcome
There are no published data about the validity of osteonecrosis diagnoses in the National Patient Register. At present, the most comprehensive validation study of this database is a review which concluded that inpatient diagnoses are 85 to 95% correct, although this figure varied among conditions and individual studies [26]. Outpatient diagnoses were not assessed in that review.
Due to the lack of validation data for osteonecrosis diagnoses, one co-author (PN) obtained the identity of all patients diagnosed with osteonecrosis (ICD-10 M87) at the University Hospital of Umeå, Sweden. For a sample of 30 patients, medical records were reviewed to validate the osteonecrosis diagnosis and to identify possible causes. The 30 sampled patients were aged 50 years or older, and they were the last 30 to be diagnosed with osteonecrosis at the hospital in 2016.
Risk factors
We collected data about diagnoses, medical procedures, surgical procedures, and prescription medications that are potential risk factors for osteonecrosis according to highly cited review articles [1, 2, 11,12,13]. We used the National Patient Register to collect data about medical procedures, surgical procedures, and diagnoses apart from cancer. Data about cancer were obtained from the Swedish Cancer Registry, which records all cases of cancer diagnosed in Sweden since 1958 [27]. Data about prescription medications were obtained from the Prescribed Drug Register, a database that records prescription medications collected at pharmacies in Sweden since July 2005 [28].
Supplemental Table 1 provides definitions of all included risk factors, in terms of ICD codes, Anatomical Therapeutic Chemical codes, and Klassifikation av kirurgiska åtgärder 1997 (Classification of Surgical Procedures 1997) codes. Supplemental Table 1 also provides information about the years for which the data were available.
Statistical analysis
Person-time at risk for osteonecrosis was the number of days from baseline (31 December 2005) until osteonecrosis diagnosis, death, emigration from Sweden, or study endpoint (31 December 2015), whichever came first. Deaths were tracked using the Cause of Death Register [29], and emigrations were tracked using the Register of the Total Population [25]. Subjects who were diagnosed with osteonecrosis prior to baseline did not contribute person-time.
Person-time at risk was grouped according to risk factors for osteonecrosis. For medical conditions, medical procedures, and surgical procedures, a subject contributed person-time from the date of his or her first diagnosis or procedure (or baseline if this came first). For hereditary, congenital, and developmental conditions, a subject contributed person-time from baseline irrespective of the date of diagnosis. For prescription medications, a subject contributed person-time on dates within 183 days (6 months) of a previously dispensed medication. A subject could contribute person-time to more than one risk factor.
We used standardized incidence ratios to compare the incidence rate of osteonecrosis in risk groups to the incidence rate in the general population. These ratios were indirectly standardized for sex and age (50–54, 55–59, …, ≥ 100 years), with the risk groups serving as standards. We did not control for confounders other than age and sex because we did not try to estimate causal effects. One limitation of standardized incidence ratios is that they are not always comparable, as the use of different standards can lead to residual confounding [30]. Despite this limitation, we chose to use risk groups as standards, instead of the general population, to reduce statistical variability and to ensure that standardized incidence ratios could be defined even in small risk groups in which not all age groups were represented [30].
We also calculated age-specific incidence rates. These rates were directly standardized for calendar year to control for changes in incidence rates and diagnostic practices over time; the overall population served as standard [30]. Confidence intervals (CIs) for incidence rates and standardized incidence ratios were estimated using Byar’s approximation [31].
Dates of events (diagnoses, medication dispensations, births, deaths, and emigrations) were occasionally missing, invalid, or incomplete (Supplemental Table 2). These events were analyzed as occurring on the earliest possible dates. Therefore, events for which the day was missing were assumed to occur on the first of the month. Events for which the month was missing were assumed to occur in January. Events for which the year was missing were excluded to prevent serious misclassification of person-time. All statistical analyses were performed in R (Version 3.4.3) and RStudio (Version 1.1.383).
Data linkage and ethics approval
Individual data were linked across databases using personal identity numbers. A personal identity number is issued by the Swedish Tax Agency to each resident of Sweden upon birth or immigration. For privacy reasons, we obtained data files in which personal identity numbers had been replaced by randomly generated identifiers, created by Statistics Sweden.
This study was conducted according to the Declaration of Helsinki, and it was approved by the Regional Ethical Review Board in Umeå, Sweden (Dnr 2013-86-31M, 2013-304-32M, and 2017-100-32M). Informed consent was not obtained because this requirement was waived by the Review Board.
Results
Study cohort and person-time at risk
Data were available for 3,347,958 residents of Sweden aged 50 years or older. We excluded the data of subjects who had a suspected incorrect personal identity number (n = 199), had a non-unique personal identity number because it was taken from a deceased person (n = 3467), or had an out-of-range date of birth (n = 1) or date of death (n = 52). With these exclusions, the study cohort comprised 3,344,239 individuals. Women constituted 53.0% (n = 1,771,035) of the cohort, and the median age at baseline was 63.9 years (interquartile range, 57.2 to 74.5 years).
The baseline prevalence of osteonecrosis was 0.17% (n = 5776); it was 0.12% (n = 1864) among men and 0.22% (n = 3912) among women. These prevalent cases were excluded from further analyses, and the remaining 3,338,463 subjects contributed 28,862,867 person-years at risk. Of those who did not develop osteonecrosis during the study, 73.6% (n = 2,448,858) were at risk for the full 10 years of follow-up, 25.4% (n = 845,094) died, and 0.9% (n = 31,086) emigrated from Sweden.
Osteonecrosis in the general population
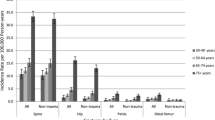
The 10-year risk of osteonecrosis was 0.40% (n = 13,425) in the general population, 0.30% (n = 4713) in men, and 0.49% (n = 8712) in women. The incidence rate of osteonecrosis was 4.7 cases/10000 person-years (95% CI, 4.6 to 4.7 cases). Among men, the incidence rate was 3.5 cases/10000 person-years (95% CI, 3.4 to 3.6 cases). Among women, it was 5.7 cases/10000 person-years (95% CI, 5.6 to 5.8 cases). The incidence rate increased with age, although the 10-year risk was approximately constant due to fewer person-years at risk in older age groups (Table 1).
Osteonecrosis was diagnosed in outpatient care in 69.0% (n = 9268) of cases. The most commonly affected skeletal sites were the femur (46.2%), knee or lower leg (16.4%), foot or ankle (7.4%), and shoulder (4.5%). The affected site was unspecified in 23.4% of cases. Most cases were classified as idiopathic (56.6%) or due to trauma (25.2%); the rest were classified as due to cause unspecified (14.9%), medication (1.9%), or disease (0.4%). Table 2 provides additional information about these diagnoses.
Osteonecrosis in risk groups
The incidence of osteonecrosis was higher in almost all risk groups than in the general population (Table 3). For some small risk groups, such as for people with decompression sickness, incidence rates and standardized incidence ratios were not meaningful because confidence intervals were too wide. Apart from decompression sickness, the standardized incidence ratio was highest in people with a previous hip fracture (7.98; 95% CI, 7.69–8.27), who constituted 21.7% of cases of osteonecrosis. Standardized incidence ratios were also high in people with osteomyelitis (6.43; 95% CI, 5.70–7.23) and in people who had undergone dialysis (6.65; 95% CI, 5.62–7.81) or solid organ transplantation (7.14; 95% CI, 5.59–8.99). Osteoarthritis was the most common comorbidity, present in 47.6% of people diagnosed with osteonecrosis. However, the standardized incidence ratio was not higher in this group than in groups with other musculoskeletal or connective tissue disorders (Table 3).
The standardized incidence ratio was higher in people who had undergone radiotherapy (3.03; 95% CI, 2.80–3.27) than in people who had been diagnosed with cancer in general (1.40; 95% CI, 1.36–1.45). The standardized incidence ratio was higher in people with systemic corticosteroid use in the last 6 months (2.61; 95% CI, 2.50–2.73) than in people with non-systemic corticosteroid use in the last 6 months (1.15; 95% CI, 1.11–1.20).
Validation of osteonecrosis diagnoses
Radiographs, taken with plain radiography or computed tomography, were available for all 30 reviewed osteonecrosis diagnoses. Twenty-seven (90%) of these diagnoses were judged correct, 2 (7%) were judged probably correct, and 1 (3%) was judged incorrect. Of the correct diagnoses, 9 were related to musculoskeletal disease (6 osteoarthritis, 1 rheumatoid arthritis, 1 reactive arthropathy, and 1 polymyalgia rheumatica), 8 to cancer or cancer therapy (e.g., radiotherapy, chemotherapy, or corticosteroids), 7 to fracture (including 1 idiopathic fracture of the acetabulum), 2 to osteoporosis medication (zoledronic acid), and 1 to surgery for hallux valgus. Of the probably correct diagnoses, 1 was related to hip fracture and 1 to proximal tibia fracture and knee osteoarthritis. The 1 incorrect diagnosis was a case of critical ischemia in a toe, related to severe atherosclerosis, but radiographs showed no sign of osteonecrosis.
Discussion
Among older adults in Sweden, the incidence of osteonecrosis was 4.7 cases/10000 person-years. The strongest risk factors for osteonecrosis were dialysis, osteomyelitis, hip fracture, and solid organ transplantation. Few previous studies have estimated the incidence of osteonecrosis in a general population, but two studies that did, one in Denmark and one in the UK, showed yearly rates of 1/10th or less than our study did [14, 15]. Our cohort was older, but this difference cannot explain the entire difference because the UK study showed a considerably lower incidence in older adults as well [15]. Possible explanations for the difference in incidence include geographic variation, a greater propensity of Swedish physicians to diagnose osteonecrosis, and a more complete coverage of specialist health care in the database we used to identify osteonecrosis, although the Danish study used a similar national database [14].
The current study showed a history of hip fracture in 22% of older adults diagnosed with osteonecrosis at any skeletal site. Previous large, population-based studies of osteonecrosis did not report the prevalence of hip fracture [14, 15], and a single-center study of osteonecrosis of the femoral head attributed only 13% of cases to hip fractures [22]. Hip fracture is a well-known risk factor for osteonecrosis of the femoral head, and the risk varies depending on the type of hip fracture and surgical procedure [32, 33]. Unfortunately, our study could not adequately distinguish between osteonecrosis of the femoral head and other types of osteonecrosis, as the skeletal site was often unspecified. Nevertheless, it is clear that a large percentage of people diagnosed with osteonecrosis at some skeletal site had a history of hip fracture.
Dialysis, osteomyelitis, and solid organ transplantation were also associated with a high incidence of osteonecrosis, but these risk factors were only present in 0.5 to 2% of cases of osteonecrosis. A previous population-based study showed a similar result for solid organ transplantation [15], but this study did not examine osteomyelitis and it examined dialysis only in combination with renal failure. Osteonecrosis is part of the pathology of osteomyelitis [34], but it is less clear why dialysis and solid organ transplantation are associated with a high risk. One explanation is the use of high-dose corticosteroids in patients undergoing solid organ transplantation [12]. Another explanation, which has been suggested for both dialysis and solid organ transplantation, is increased secretion of parathyroid hormone [12]. Our study indicates that dialysis, osteomyelitis, and solid organ transplantation do not contribute substantially to the burden of osteonecrosis in the general population. However, our results also indicate that osteonecrosis is a real concern in these risk groups.
As with solid organ transplantation, many medical conditions may be associated with osteonecrosis due to the use of systemic corticosteroids [12]. The present study showed that systemic corticosteroid use in the last 6 months was a strong, but not one of the strongest, risk factors for osteonecrosis. It was present in 15% of cases, which is similar to the results of some previous studies [15, 19]. However, studies of non-traumatic osteonecrosis of the femoral head have attributed 50% or more of cases to corticosteroids [16,17,18]. Our results may not be generalizable to ever or high-dose corticosteroid users or to patients with non-traumatic osteonecrosis, but our results suggest that recent corticosteroid use can explain at most a minority of cases of osteonecrosis.
Previous studies have shown a high risk of osteonecrosis in people with systemic lupus erythematosus [35, 36]. In our study, this disease was not a stronger risk factor for osteonecrosis than were several other musculoskeletal and connective tissue diseases. Data are lacking from other population-based studies to verify this result. It may apply only to older adults, as systemic lupus erythematosus tends to develop in younger adults [36].
Our study showed that almost 50% of people diagnosed with osteonecrosis also had osteoarthritis. A large part of this high prevalence appeared to be due to a high prevalence of osteoarthritis in the general population, as osteoarthritis was not a stronger risk factor for osteonecrosis than were other musculoskeletal diseases. The analysis was only controlled for age and sex, as we did not try to determine causality, but a previous retrospective cohort study showed that osteoarthritis was one of the strongest risk factors for osteonecrosis after adjustment for multiple confounders [15]. Osteoarthritis has long been discussed as a possible cause of osteonecrosis [37, 38]. However, it is rarely listed as a risk factor in review articles [1, 2, 11,12,13], so this type of osteonecrosis could be underdiagnosed. On the other hand, osteonecrosis could be overdiagnosed in our and in previous retrospective cohort studies since the two conditions have similar symptoms [2]. To complicate matters further, osteonecrosis itself is a risk factor for osteoarthritis, as late-stage osteonecrosis is characterized by joint destruction [23]. More research is needed to determine whether osteoarthritis increases the risk of osteonecrosis. To minimize confounding and misclassification bias, this research should preferably be based on patient data that are more detailed than the data available in most retrospective cohort studies.
Five limitations of this study should be noted. First, we validated only a convenience sample of 30 osteonecrosis diagnoses made at one university hospital. Second, we did not study osteonecrosis of the jaw, which has been related to osteoporosis treatment [39], because osteonecrosis of the jaw does not have a specific ICD-10 code. Third, the prevalence of osteonecrosis was probably underestimated, as data about osteonecrosis were available only from 1997. Fourth, the prevalence of some risk factors was probably also underestimated because the National Patient Register has a low sensitivity for many conditions, especially those managed in primary care [26]. Since mild conditions are more likely to be managed in primary care than severe conditions are, our results may not be generalizable to mild conditions. Fifth, we could not completely determine the role of alcohol consumption in osteonecrosis because we only had data about a surrogate of high alcohol consumption.
In sum, this nationwide study of older adults in Sweden showed that osteonecrosis was approximately 10 times more common than a small number of previous population-based studies have suggested. The strongest risk factors for osteonecrosis were dialysis, osteomyelitis, hip fracture, and solid organ transplantation. Although this study did not attempt to estimate causal effects, only hip fractures could have contributed substantially to the disease burden, as hip fractures were present in one fifth of older adults diagnosed with osteonecrosis at various skeletal sites. Dialysis, osteomyelitis, and solid organ transplantation were rare among older adults diagnosed with osteonecrosis, but the high incidence in these groups is concerning.
References
Mankin H (1992) Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med 326:1473–1479. https://doi.org/10.1056/NEJM199205283262206
Pavelka K (2000) Osteonecrosis. Baillieres Best Pr Res Clin Rheumatol 14:399–414. https://doi.org/10.1053/berh.2000.0072
Sugano N, Masuhara K, Nakamura N, Ochi T, Hirooka A, Hayami Y (1996) MRI of early osteonecrosis of the femoral head after transcervical fracture. J Bone Jt Surg Br 78–B:253–257
Nikolopoulos K, Papadakis S, Kateros K et al (2003) Long-term outcome of patients with avascular necrosis, after internal fixation of femoral neck fractures. Injury 34:525–528. https://doi.org/10.1016/S0020-1383(02)00367-4
Hattrup SJ, Cofield RH (1999) Osteonecrosis of the humeral head: relationship of disease stage, extent, and cause to natural history. J Soulder Elb Surg 8:559–564. https://doi.org/10.1016/S1058-2746(99)90089-7
Kwon JW, Huh SJ, Yoon YC, Choi SH, Jung JY, Oh D, Choe BK (2008) Pelvic bone complications after radiation therapy of uterine cervical cancer: evaluation with MRI. AJR Am J Roentgenol 191:987–994. https://doi.org/10.2214/AJR.07.3634
Mehmood Q, Beardwood M, Swindell R, Greenhalgh S, Wareham T, Barraclough L, Livsey J, Davidson SE (2014) Insufficiency fractures in patients treated with pelvic radiotherapy and chemotherapy for uterine and cervical cancer. Eur J Cancer Care (Engl) 23:43–50. https://doi.org/10.1111/ecc.12105
Saito M, Ueshima K, Fujioka M, Ishida M, Goto T, Arai Y, Ikoma K, Fujiwara H, Fukushima W, Kubo T (2014) Corticosteroid administration within 2 weeks after renal transplantation affects the incidence of femoral head osteonecrosis. Acta Orthop 85:266–270. https://doi.org/10.3109/17453674.2014.916490
Nagasawa K, Tada Y, Koarada S, Horiuchi T, Tsukamoto H, Murai K, Ueda A, Yoshizawa S, Ohta A (2005) Very early development of steroid-associated osteonecrosis of femoral head in systemic lupus erythematosus: prospective study by MRI. Lupus 14:385–390. https://doi.org/10.1191/0961203305lu2103oa
Orlić D, Jovanović D, Antičević D, Zečević J (1990) Frequency of idiopathic aseptic necrosis in medically treated alcoholics. Int Orthop 14:383–386
Assouline-Dayan Y, C C, A G et al (2002) Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 32:94–124. https://doi.org/10.1053/sarh.2002.33724
Mirzai R, Chang C, Greenspan A, Gershwin ME (1999) The pathogenesis of osteonecrosis and the relationships to corticosteroids. J Asthma 36:77–95. https://doi.org/10.3109/02770909909065152
Lafforgue P (2006) Pathophysiology and natural history of avascular necrosis of bone. Joint Bone Spine 73:500–507. https://doi.org/10.1016/j.jbspin.2006.01.025
Dima A, Pedersen AB, Pedersen L, Baicus C, Thomsen RW (2018) Association of common comorbidities with osteonecrosis: a nationwide population-based case–control study in Denmark. BMJ Open 8:e020680. https://doi.org/10.1136/bmjopen-2017-020680
Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB (2010) The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int 21:569–577. https://doi.org/10.1007/s00198-009-1003-1
Ikeuchi K, Hasegawa Y, Seki T, Takegami Y, Amano T, Ishiguro N (2015) Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod Rheumatol 25:278–281. https://doi.org/10.3109/14397595.2014.932038
Fukushima W, Fujioka M, Kubo T, Tamakoshi A, Nagai M, Hirota Y (2010) Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res 468:2715–2724. https://doi.org/10.1007/s11999-010-1292-x
Zhao DW, Yu M, Hu K, Wang W, Yang L, Wang BJ, Gao XH, Guo YM, Xu YQ, Wei YS, Tian SM, Yang F, Wang N, Huang SB, Xie H, Wei XW, Jiang HS, Zang YQ, Ai J, Chen YL, Lei GH, Li YJ, Tian G, Li ZS, Cao Y, Ma L (2015) Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J 128:2843–2850. https://doi.org/10.4103/0366-6999.168017
Kang JS, Park S, Song H et al (2009) Prevalence of osteonecrosis of the femoral head: a nationwide epidemiologic analysis in Korea. J Arthroplasty 24:1178–1183. https://doi.org/10.1016/j.arth.2009.05.022
Abbott KC, Koff J, Bohen EM, Oglesby RJ, Agodoa LYC, Lentine KL, Schnitzler MA (2005) Maintenance immunosuppression use and the associated risk of avascular necrosis after kidney transplantation in the United States. Transplantation 79:330–336. https://doi.org/10.1097/01.TP.0000149894.95435.7F
Bin JY, Sung Y-K, Shim J-S et al (2015) Prevalence, incidence, and associated factors of avascular necrosis in Korean patients with systemic lupus erythematosus: a nationwide epidemiologic study. Rheumatol Int 35:879–886. https://doi.org/10.1007/s00296-014-3147-3
Urbaniak JR, Coogan PG, Gunneson EB, Nunley JA (1995) Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting. A long-term follow-up study of one hundred and three hips. J Bone Jt Surg 77:681–694
Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, Kadowaki T (1991) Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br 73–B 73-B:68–72
L’Insalata JC, Pagnani MJ, Warren RF, Dines DM (1996) Humeral head osteonecrosis: clinical course and radiographic predictors of outcome. J Shoulder Elb Surg 5:355–361. https://doi.org/10.1016/S1058-2746(96)80066-8
Statistics Sweden. Individregister och mikrodata. https://www.scb.se/vara-tjanster/bestalla-mikrodata/vilka-mikrodata-finns/individregister/. Accessed 1 Mar 2018
Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO (2011) External review and validation of the Swedish national inpatient register. BMC Public Health 11(450). https://doi.org/10.1186/1471-2458-11-450
Swedish National Board of Health and Welfare. Swedish Cancer Registry. http://www.socialstyrelsen.se/register/halsodataregister/cancerregistret/inenglish. Accessed 19 Apr 2018
Wettermark B, Hammar N, MichaelFored C, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundström A, Westerholm B, Rosén M (2007) The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 16:726–735. https://doi.org/10.1002/pds
Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R (2017) The Swedish cause of death register. Eur J Epidemiol 32:765–773. https://doi.org/10.1007/s10654-017-0316-1
Szklo M, Nieto FJ (2014) Epidemiology: beyond the basics, 3rd edn. Jones & Bartlett Learning, Burlington
Breslow NE, Day NE (1987) Statistical methods in cancer research: volume II - the design and analysis of cohort studies. International Agency for Research on Cancer, Lyon
Swiontkowski MF (1994) Intracapsular fractures of the hip. J bone Jt Surg 76:129–138
Bhandari M, Devereaux PJ, Swiontkowski MF et al (2003) Internal fixation compared with arthroplasty for displaced fractures of the femoral neck: a meta-analysis. J Bone Jt Surg Am 85–A:1673–1681. https://doi.org/10.2106/00004623-200309000-00004
Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364:369–379. https://doi.org/10.1016/S0140-6736(04)16727-5
Zonana-Nacach A, Barr SG, Magder LS, Petri M (2000) Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum 43:1801–1808. https://doi.org/10.1002/1529-0131(200008)43:8<1801::AID-ANR16>3.0.CO;2-O
Mok M, Farewell V, Isenberg D (2000) Risk factors for avascular necrosis of bone in patients with systemic lupus erythematosus: is there a role for antiphospholipid antibodies? Ann Rheum Dis 59:462–467
Ilardi CF, Sokoloff L (1984) Secondary osteonecrosis in osteoarthritis of the femoral head. Hum Pathol 15:79–83. https://doi.org/10.1016/S0046-8177(84)80334-2
Yamamoto T, Yamaguchi T, Lee KB, Bullough PG (2000) A clinicopathologic study of osteonecrosis in the osteoarthritic hip. Osteoarthr Cartil 8:303–308. https://doi.org/10.1053/joca.1999.0305
Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, al Dabagh R, Davison KS, Kendler DL, Sándor GK, Josse RG, Bhandari M, el Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J, on behalf of the International Task Force on Osteonecrosis of the Jaw (2015) Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res 30:3–23. https://doi.org/10.1002/jbmr.2405
Funding
This study was funded by Vetenskapsrådet (Swedish Research Council) (grant number 2016-02584).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 49 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bergman, J., Nordström, A. & Nordström, P. Epidemiology of osteonecrosis among older adults in Sweden. Osteoporos Int 30, 965–973 (2019). https://doi.org/10.1007/s00198-018-04826-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-04826-2