Abstract
Summary
Higher levels of Dickkopf-1, which is an inhibitor of Wnt/β-catenin bone metabolic pathway, could be indicative of downregulated Wnt system, with possible lower osteoblast activation and higher osteoclast signaling in type 1 diabetes mellitus children and adolescents. Dickkopf-1 could significantly contribute to diabetes osteopathy.
Introduction
Increased fracture risk and elevated Dickkopf-1 levels, which is an inhibitor of Wnt/β-catenin bone metabolic pathway, have been documented in adult patients with type 2 diabetes mellitus (T2D), while no relevant data exist on childhood type 1 diabetes (T1D). Our aim was to study plasma Dickkopf-1 distribution in children and adolescents with T1D and to correlate Dickkopf-1 with metabolic bone markers and bone mineral density (BMD).
Methods
We evaluated 40 children and adolescents with T1D (mean ± SD age 13.04 ± 3.53 years, T1D duration 5.15 ± 3.33 years) and 40 healthy age-matched and gender-matched controls (age 12.99 ± 3.3 years). Dickkopf-1 and bone metabolic markers were measured, while total body and lumbar spine BMD were evaluated with dual-energy X-ray absorptiometry (DXA).
Results
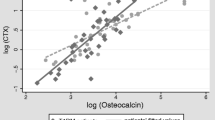
Dickkopf-1 demonstrated a Gaussian distribution, with higher levels in T1D patients (13.56 ± 5.34 vs 11.35 ± 3.76 pmol/L, p = 0.024). Higher values were found in boys and in prepubertal children. Dickkopf-1 correlated positively with osteoprotegerin and fasting glucose in patients, while positive correlation with sclerostin and total soluble receptor activator of nuclear factor-kappaB ligand (s-RANKL) was found in controls. Positive correlations with C-telopeptide cross-links (CTX), osteocalcin, alkaline phosphatase, phosphate, and insulin-like growth factor 1 (IGF1) were documented in both groups. Lumbar spine Z-score was positively associated with Dickkopf-1 in controls, while a negative trend was found in patients.
Conclusions
Higher levels of Dickkopf-1 could indicate a downregulated Wnt/β-catenin system with possible lower osteoblast activation and higher osteoclast signaling in T1D children and adolescents. Dickkopf-1 could possibly be a significant contributor of T1D osteopathy. Future therapies could focus on Wnt/β-catenin metabolic pathway.




Similar content being viewed by others
References
Rakel A, Sheehy O, Rahme E, LeLorier J (2008) Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab 34:193–205
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 18:427–444
Khan T, Fraser L (2015) Type 1 diabetes and osteoporosis: from molecular pathways to bone phenotype. J Osteoporos 2015:174186
Starup-Linde J (2013) Diabetes, biochemical markers of bone turnover, diabetes control, and bone. Front Endocrinol 4:21
Roy B (2013) Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J Diabetes 4:101–113
Yavropoulou MP, Yovos JG (2007) The role of the wnt signaling pathway in osteoblast commitment and differentiation. Hormones 6:279–294
Nishimura R, Hata K, Ikeda F, Ichida F, Shimoyama A, Matsubara T, Wada M, Amano K, Yoneda T (2008) Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J Bone Miner Metab 26:203–212
Krishnan V, Bryant HU, MacDougald OA (2006) Regulation of bone mass by wnt signaling. J Clin Invest 116:1202–1209
MacDonald B, Joiner D, Oyserman S, Sharma P, Goldstein S, He X, PV. H (2007) Bone mass is inversely proportional to Dkk1 levels in mice. Bone 41:331–339
Grotewold L, Ruther U (2002) THe wnt antagonist dickkopf-1 is regulated by bmp signaling and c-Jun and modulates programmed cell death. EMBO J 21:966–975
Choi HY, Dieckmann M, Herz J, Niemeier A (2009) Lrp4, a novel receptor for dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One 4:e7930
Balemans W, Piters E, Cleiren E, m A, Van Wesenbeeck L, Warman M, Van Hul W (2008) The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone nass LRP5. Calcif Tissue Int 82:445–453
Gaur T, Lengner CJ, Hovhannisyan H et al (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 Gene expression. J Biol Chem 280:33132–33140
Fowlkes J, Bunn R, Liu L, Wahl E, Coleman H, Cockrell G, Perrien D, Lumpkin CJ, Thrailkill K (2008) Runt-related transcription factor 2 (RUNX2) and RUNX2-related Osteogenic genes are down-regulated throughout osteogenesis in type 1 diabetes mellitus. Endocrinology 149:1697–1704
Portal-Nunez S, Lozano D, de Castro LF, de Gortazar AR, Nogues X, Esbrit P (2010) Alterations of the wnt/beta-catenin pathway and its target genes for the N- and C-terminal domains of parathyroid hormone-related protein in bone from diabetic mice. FEBS Lett 584:3095–3100
Hie M, Iitsuka N, Otsuka T, Tsukamoto I (2011) Insulin-dependent diabetes mellitus decreases osteoblastogenesis associated with the inhibition of wnt signaling through increased expression of Sost and Dkk1 and inhibition of Akt activation. Int J Mol Med 28:455–462
Gaudio A, Privitera F, Pulvirenti I, Canzonieri E, Rapisarda R, Fiore CE (2014) The relationship between inhibitors of the wnt signalling pathway (sclerostin and dickkopf-1) and carotid intima-media thickness in postmenopausal women with type 2 diabetes mellitus. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease 11:48–52
Garcia-Martin A, Reyes-Garcia R, Garcia-Fontana B, Morales-Santana S, Coto-Montes A, Munoz-Garach M, Rozas-Moreno P, Munoz-Torres M (2014) Relationship of Dickkopf1 (DKK1) with cardiovascular disease and bone metabolism in Caucasian type 2 diabetes mellitus. PLoS One 9:e111703
Lattanzio S, Santilli F, Liani R, Vazzana N, Ueland T, Di Fulvio P, Formoso G, Consoli A, Aukrust P, Davi G (2014) Circulating dickkopf-1 in diabetes mellitus: association with platelet activation and effects of improved metabolic control and low-dose aspirin. J Am Heart Assoc 3
Register TC, Hruska KA, Divers J et al (2013) Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J Clin Endocrinol Metab 98:E60–E65
Qiu F, He J, Zhou Y, Bai X, Wu G, Wang X, Liu Z, Chen Y, Ma JX, Liu Z (2014) Plasma and vitreous fluid levels of dickkopf-1 in patients with diabetic retinopathy. Eye 28:402–409
Tsentidis C, Gourgiotis D, Kossiva L, Marmarinos A, Doulgeraki A, Karavanaki K (2016) Sclerostin distribution in children and adolescents with type 1 diabetes mellitus and correlation with bone metabolism and bone mineral density. Pediatr Diabetes 17:289–299
Tsentidis C, Gourgiotis D, Kossiva L, Doulgeraki A, Marmarinos A, Galli-Tsinopoulou A, Karavanaki K (2016) Higher levels of s-RANKL and osteoprotegerin in children and adolescents with type 1 diabetes mellitus may indicate increased osteoclast signaling and predisposition to lower bone mass: a multivariate cross-sectional analysis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27:1631–1643
Gustafson B, Eliasson B, Smith U (2010) Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor dickkopf-1 in adipocytes: a link with osteogenesis. Diabetologia 53:536–540
Ahmed SF, Fouda N, Abbas AA (2013) Serum dickkopf-1 level in postmenopausal females: correlation with bone mineral density and serum biochemical markers. J Osteoporos 2013:460210
Anastasilakis AD, Polyzos SA, Avramidis A, Toulis KA, Papatheodorou A, Terpos E (2010) The effect of teriparatide on serum dickkopf-1 levels in postmenopausal women with established osteoporosis. Clin Endocrinol 72:752–757
Voskaridou E, Christoulas D, Xirakia C, Varvagiannis K, Boutsikas G, Bilalis A, Kastritis E, Papatheodorou A, Terpos E (2009) Serum dickkopf-1 is increased and correlates with reduced bone mineral density in patients with thalassemia-induced osteoporosis. Reduction post-zoledronic acid administration Haematologica 94:725–728
Brunetti G, Faienza M, Piacente L et al (2013) High dickkopf-1 levels in sera and leukocytes from children with 21-hydroxylase deficiency on chronic glucocorticoid treatment. Am J Physiol Endocrinol Metab 304:E546–E554
Mak W, Shao X, Dunstan C, Seibel M, Zhou H (2009) Biphasic glucocorticoid-dependent regulation of wnt expression and its inhibitors in mature osteoblastic cells. Calcif Tissue Int 85:538–545
Butler JS, Queally JM, Devitt BM, Murray DW, Doran PP, O’Byrne JM (2010) Silencing Dkk1 expression rescues dexamethasone-induced suppression of primary human osteoblast differentiation. BMC Musculoskelet Disord 11:210
Sottnik JL, Hall CL, Zhang J, Keller ET (2012) Wnt and wnt inhibitors in bone metastasis. BoneKEy reports 1:101
Hameed A, Brady JJ, Dowling P, Clynes M, O’Gorman P (2014) Bone disease in multiple myeloma: pathophysiology and management. Cancer growth and metastasis 7:33–42
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Rights and permissions
About this article
Cite this article
Tsentidis, C., Gourgiotis, D., Kossiva, L. et al. Increased levels of Dickkopf-1 are indicative of Wnt/β-catenin downregulation and lower osteoblast signaling in children and adolescents with type 1 diabetes mellitus, contributing to lower bone mineral density. Osteoporos Int 28, 945–953 (2017). https://doi.org/10.1007/s00198-016-3802-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3802-5