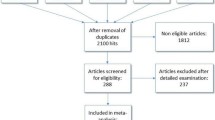
Abstract
Purpose
Hydroxyethyl starch (HES) is a synthetic colloid used widely for resuscitation despite the availability of safer, less costly fluids. Numerous HES reviews have been published that may have influenced clinicians’ practice. We have therefore examined the relationship between the methodological quality of published HES reviews, authors’ potential conflicts of interest (pCOI) and the recommendations made.
Methods
Systematic analysis of reviews on HES use.
Results
Between 1975 and 2010, 165 reviews were published containing recommendations for or against HES use. From the 1990s onwards, favorable reviews increased from two to eight per year and HES’s share of the artificial colloid market tripled from 20 to 60 %. Only 7 % (12/165) of these reviews of HES use contained meta-analyses; these 7 % had higher Overview Quality Assessment Questionnaire (OQAQ) scores [median (range) 6.5 (3–7)] than reviews without meta-analysis [2 (1–4); p < 0.001]. The rates of recommending against HES use are 83 % (10/12) in meta-analyses and 20 % (31/153) in reviews without meta-analysis (p < 0.0001). Fourteen authors published the majority (70/124) of positive reviews, and ten of these 14 had or have since developed a pCOI with various manufacturers of HES.
Conclusions
Low-quality HES reviews reached different conclusions than high-quality meta-analyses from independent entities, such as Cochrane Reviews. The majority of these low-quality positive HES reviews were written by a small group of authors, most of whom had or have since established ties to industry. The proliferation of positive HES reviews has been associated with increased utilization of an expensive therapy despite the lack of evidence for meaningful clinical benefit and increased risks. Clinicians need to be more informed that marketing efforts are potentially influencing scientific literature.





Similar content being viewed by others
References
Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, Du B, McArthur C, Myburgh J, Investigators TS (2010) Resuscitation fluid use in critically ill adults: an international cross sectional study in 391 intensive care units. Critical Care (Lond) 14:R185
Schortgen F, Deye N, Brochard L (2004) Preferred plasma volume expanders for critically ill patients: results of an international survey. Intensive Care Med 30:2222–2229
The FLUIDS study investigators for the Scandinavian Critical Care Trials Group (2008) Preferences for colloid use in Scandinavian intensive care units. Acta Anaesthesiol Scand 52:750-758
Basora M, Moral V, Llau JV, Silva S (2007) Perioperative colloid administration: a survey of Spanish anesthesiologists’ attitudes. Rev Esp Anestesiol Reanim 54:162–168
Liu FC, Liao CH, Chang YW, Liou JT, Day YJ (2009) Hydroxyethyl starch interferes with human blood ex vivo coagulation, platelet function and sedimentation. Acta Anaesthesiol Taiwan 47:71–78
Hartog CS, Brunkhorst FM, Bloos F, Bogatsch H, Engel C, Sengebusch K, Reinhart K, Ragaller M (2010) Practice of volume therapy in patients with severe sepsis: results from a nationwide sepsis prevalence study. Intensive Care Med 36:553–554
Westphal M, James MF, Kozek-Langenecker S, Stocker R, Guidet B, Van Aken H (2009) Hydroxyethyl starches: different products—different effects. Anesthesiology 111:187–202
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K (2008) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 358:125–139
Zarychanski R, Turgeon AF, Fergusson DA, Cook DJ, Hebert P, Bagshaw SM, Monsour D, McIntyre LA (2009) Renal outcomes and mortality following hydroxyethyl starch resuscitation of critically ill patients: systematic review and meta-analysis of randomized trials. Open Med 3:E196–E209
Dart AB, Mutter TC, Ruth CA, Taback SP (2010) Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database of Systematic Reviews (Online):CD007594
Wilkes MM, Navickis RJ, Sibbald WJ (2001) Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg 72:527–533
Perel P, Roberts I (2009) Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database of Systematic Reviews (Online):CD000567
Bunn F, Trivedi D, Ashraf S (2008) Colloid solutions for fluid resuscitation. Cochrane Database of Systematic Reviews (Online):CD001319
Mishler JM (1984) Synthetic plasma volume expanders—their pharmacology, safety and clinical efficacy. Clin Haematol 13:75–92
Vincent JL (1991) Fluids for resuscitation. Br J Anaesth 67:185–193
Treib J, Baron JF, Grauer MT, Strauss RG (1999) An international view of hydroxyethyl starches. Intensive Care Med 25:258–268
de Jonge E, Levi M (2001) Effects of different plasma substitutes on blood coagulation: a comparative review. Crit Care Med 29:1261–1267
Kozek-Langenecker SA (2005) Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology 103:654–660
Boldt J (2009) Modern rapidly degradable hydroxyethyl starches: current concepts. Anesth Analg 108:1574–1582
McAlister FA, Graham I, Karr GW, Laupacis A (1999) Evidence-based medicine and the practicing clinician. J Gen Intern Med 14:236–242
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 354:1896–1900
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J (Clinical Research edn) 339:b2700
Guyatt GH, Haynes RB, Jaeschke RZ, Cook DJ, Green L, Naylor CD, Wilson MC, Richardson WS (2000) Users’ Guides to the Medical Literature: XXV. Evidence-based medicine: principles for applying the Users’ Guides to patient care. Evidence-based medicine working group. JAMA 284:1290–1296
Loke YK, Derry S (2003) Does anybody read “evidence-based” articles? BMC Med Res Methodol 3:14
Cook DJ, Mulrow CD, Haynes RB (1997) Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med 126:376–380
Schmidt LM, Gotzsche PC (2005) Of mites and men: reference bias in narrative review articles: a systematic review. J Fam Pract 54:334–338
Oxman AD, Guyatt GH, Singer J, Goldsmith CH, Hutchison BG, Milner RA, Streiner DL (1991) Agreement among reviewers of review articles. J Clin Epidemiol 44:91–98
Shikowitz MJ (1991) Sudden sensorineural hearing loss. Med Clin N Am 75:1239–1250
Heilmann L, Siekmann U, Schmid-Schonbein H (1985) The rheologic properties of the blood during pregnancy. Dtsch Med Wochenschr 110:1705–1708
Laatikainen LT (1992) Management of retinal vein occlusion. Curr Opin Ophthalmol 3:372–378
Aboulghar MA, Mansour RT, Aboulghar MA, Mansour RT (2003) Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Hum Reprod Update 9:275–289
Forouzandeh B, Konicek F, Sheagren JN (1996) Large-volume paracentesis in the treatment of cirrhotic patients with refractory ascites. The role of postparacentesis plasma volume expansion. J Clin Gastroenterol 22:207–210
Berger W, Keller U, Berger W, Keller U (1992) Treatment of diabetic ketoacidosis and non-ketotic hyperosmolar diabetic coma. Baillieres Clin Endocrinol Metab 6:1–22
Leschke M, Klimek W, Jung F (2003) Rheological determinants of end-organ damage. Der Internist 44:853–863
Linde I (1993) Indications and contraindications of plasmapheresis and autologous blood donation. Beitr Infusionstherap (Contributions to infusion therapy) 29:158–162
Nusbacher J, McCullough J, Huestis DW (1977) Granulocyte collection and processing. Prog Clin Biol Res 13:175–183
Southard JH, Belzer FO (1995) Organ preservation. Annu Rev Med 46:235–247
Raju GM, Kochupillai V, Kumar L (1995) Storage of haemopoietic stem cells for autologous bone marrow transplantation. Natl Med J India 8:216–221
Kreimeier U, Christ F, Frey L, Habler O, Thiel M, Welte M, Zwissler B, Peter K (1997) Small-volume resuscitation for hypovolemic shock. Concept, experimental and clinical results. Der Anaesthesist 46:309–328
Delaney A, Bagshaw SM, Ferland A, Manns B, Laupland KB, Doig CJ (2005) A systematic evaluation of the quality of meta-analyses in the critical care literature. Critical Care (London, England) 9:R575–R582
Choi PT, Halpern SH, Malik N, Jadad AR, Tramer MR, Walder B (2001) Examining the evidence in anesthesia literature: a critical appraisal of systematic reviews. Anesth Analg 92:700–709
Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, Henry DA, Boers M (2009) AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 62:1013–1020
Nolan J (1999) Fluid replacement. Br Med Bull 55:821–843
Artificial colloids. Market analysis. IMS Health (2009)
Barron ME, Wilkes MM, Navickis RJ (2004) A systematic review of the comparative safety of colloids. Arch Surg 139:552–563
Thompson WL (1975) Rational use of albumin and plasma substitutes. Johns Hopkins Med J 136:220–225
Boldt J (2006) Volume therapy in cardiac surgery: are Americans different from Europeans? J Cardiothorac Vasc Anesth 20:98–105
Gosling P, Rittoo D, Manji M, Mahmood A, Vohra R (2001) Hydroxyethylstarch as a risk factor for acute renal failure in severe sepsis. Lancet 358:581 author reply 582
Boldt J (2006) Do plasma substitutes have additional properties beyond correcting volume deficits? Shock (Augusta Ga) 25:103–116
Zikria BA, King TC, Stanford J, Freeman HP (1989) A biophysical approach to capillary permeability. Surgery 105:625–631
Boldt J (2008) Saline versus balanced hydroxyethyl starch: does it matter? Curr Opin Anaesthesiol 21:679–683
Boldt J, Ince C (2010) The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: a review. Intensive Care Med 36:1299–1308
Adams HA, Vogt PM (2009) Circulation therapy for severe burn injuries. Der Unfallchirurg 112:462–471
Powell-Tuck J, Gosling P, Lobo DN, Allison S, Carlson GL, Gore M, Lewington AJ, Pearse RM, Mythen MG, (2008) British consensus guidelines on intravenous fluid therapy for adult surgical patients (GIFTASUP). 2008. London: NHS National Library of Health. Available at: http://www.ics.ac.uk/downloads/2008112340_GIFTASUP%20FINAL_31-10-08.pdf doi
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
The American Thoracic Society Colloid Working Group (2004) Evidence-based colloid use in the critically ill: American Thoracic Society Consensus Statement. Am J Respir Crit Care Med 170:1247–1259
Reinhart K, Brunkhorst FM, Bone HG, Bardutzky J, Dempfle CE, Forst H, Gastmeier P, Gerlach H, Grundling M, John S, Kern W, Kreymann G, Kruger W, Kujath P, Marggraf G, Martin J, Mayer K, Meier-Hellmann A, Oppert M, Putensen C, Quintel M, Ragaller M, Rossaint R, Seifert H, Spies C, Stuber F, Weiler N, Weimann A, Werdan K, Welte T (2010) Prevention, diagnosis, treatment, and follow-up care of sepsis. First revision of the S2k Guidelines of the German Sepsis Society (DSG) and the German Interdisciplinary Association for Intensive and Emergency Care Medicine (DIVI). Der Anaesthesist 59:347–370
Joannidis M, Druml W, Forni LG, Groeneveld AB, Honore P, Oudemans-van Straaten HM, Ronco C, Schetz MR, Woittiez AJ (2009) Prevention of acute kidney injury and protection of renal function in the intensive care unit. Expert opinion of the Working Group for Nephrology, ESICM. Intensive Care Med 36:392–411
Greaves I, Porter KM, Revell MP (2002) Fluid resuscitation in pre-hospital trauma care: a consensus view. J R Coll Surg Edinb 47:451–457
Hardy JF, de Moerloose P, Samama CM (2006) Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth (J Canadien d’anesthesie) 53:S40–S58
Human albumin expert group (2000) Indications for the use of human albumin solutions: an expert report. Schweiz Med Wochenschr 130:516–522
Kruskall MS, Mintz PD, Bergin JJ, Johnston MF, Klein HG, Miller JD, Rutman R, Silberstein L (1988) Transfusion therapy in emergency medicine. Ann Emerg Med 17:327–335
Martel MJ, MacKinnon KJ, Arsenault MY, Bartellas E, Klein MC, Lane CA, Sprague AE, Wilson AK (2002) Hemorrhagic shock. J Obstet Gynaecol Can 24:504–520 quiz 521–504
Vermeulen LC Jr, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA (1995) A paradigm for consensus. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med 155:373–379
Asplund K (2009) Haemodilution for acute ischaemic stroke. Cochrane database of systematic reviews (Online):CD000103
Himpe D (2003) Colloids versus crystalloids as priming solutions for cardiopulmonary bypass: a meta-analysis of prospective, randomised clinical trials. Acta Anaesthesiol Belg 54:207–215
Cyna AM, Andrew M, Emmett RS, Middleton P, Simmons SW (2009) Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane database of systematic reviews (Online):CD002251
Toomtong P, Suksompong S (2010) Intravenous fluids for abdominal aortic surgery. Cochrane database of systematic reviews (Online):CD000991
Dretzke J, Sandercock J, Bayliss S, Burls A (2004) Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients. Health technology assessment (Winchester, England) 8(23):1–103
Kozek-Langenecker SA, Jungheinrich C, Sauermann W, Van der Linden P (2008) The effects of hydroxyethyl starch 130/0.4 (6 %) on blood loss and use of blood products in major surgery: a pooled analysis of randomized clinical trials. Anesth Analg 107:382–390
Wang AT, McCoy CP, Murad MH, Montori VM (2010) Association between industry affiliation and position on cardiovascular risk with rosiglitazone: cross sectional systematic review. Br Med J (Clinical Research edn) 340:c1344
Jorgensen AW, Hilden J, Gotzsche PC (2006) Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: systematic review. Br Med J (Clinical Research edn) 333:782
Barnes DE, Bero LA (1998) Why review articles on the health effects of passive smoking reach different conclusions. JAMA 279:1566–1570
Stelfox HT, Chua G, O’Rourke K, Detsky AS (1998) Conflict of interest in the debate over calcium-channel antagonists. N Engl J Med 338:101–106
Fugh-Berman A, McDonald CP, Bell AM, Bethards EC, Scialli AR (2011) Promotional tone in reviews of menopausal hormone therapy after the women’s health initiative: an analysis of published articles. PLoS Med 8:e1000425
Shafer SL (2010) Notice of retraction. Anesth Analg 111:1567
Editors in-Chief (2011) Editor-in Chief statement regarding published clinical trials conducted without IRB approval by Joachim Boldt. Available at: http://journals.lww.com/ejanaesthesiology/Documents/EIC%20Joint%20Statement%20on%20Retractions%2012Mar2011.pdf. Accessed 12 Mar 2011
Angell M (2000) Is academic medicine for sale? N Engl J Med 342:1516–1518
Groeneveld AB, Navickis RJ, Wilkes MM (2011) Update on the comparative safety of colloids: a systematic review of clinical studies. Ann Surg 253:470–483
Hartog CS, Kohl M, Reinhart K (2011) A systematic review of third-generation hydroxyethyl starch (HES 130/0.4) in resuscitation: safety not adequately addressed. Anesth Analg 112:635–645
Carey TS, Williams JW Jr, Oldham JM, Goodman F, Ranney LM, Whitener L, Morgan LC, Melvin CL (2008) Gabapentin in the treatment of mental illness: the echo chamber of the case series. J Psychiatr Pract 14[Suppl 1]:15–27
Murphy GS, Greenberg SB (2010) The new-generation hydroxyethyl starch solutions: the Holy Grail of fluid therapy or just another starch? J Cardiothorac Vasc Anesth 24:389–393
Landefeld CS, Steinman MA (2009) The Neurontin legacy–marketing through misinformation and manipulation. N Engl J Med 360:103–106
Prayle AP, Hurley MN, Smyth AR (2012) Compliance with mandatory reporting of clinical trial results on ClinicalTrials.gov: cross sectional study. Br Med J (Clinical Research edn) 344:d7373
National Health Service (2008) Colloids versus crystalloids for fluid resuscitation in critically ill patients. NHS evidence: quality, innovation, productivity and prevention (QIPP). Available online at: https://www.evidence.nhs.uk/qipp
Schochl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C (2010) Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care (Lond) 14:R55
Woessner R, Grauer MT, Dieterich HJ, Bepperling F, Baus D, Kahles T, Georgi S, Bianchi O, Morgenthaler M, Treib J (2003) Influence of a long-term, high-dose volume therapy with 6 % hydroxyethyl starch 130/0.4 or crystalloid solution on hemodynamics, rheology and hemostasis in patients with acute ischemic stroke. Results of a randomized, placebo-controlled, double-blind study. Pathophysiol Haemost Thromb 33:121–126
Dieterich HJ, Weissmuller T, Rosenberger P, Eltzschig HK (2006) Effect of hydroxyethyl starch on vascular leak syndrome and neutrophil accumulation during hypoxia. Crit Care Med 34:1775–1782
James MF (2008) The role of tetrastarches for volume replacement in the perioperative setting. Curr Opin Anaesthesiol 21:674–678
Vincent JL (2006) Resuscitation using albumin in critically ill patients: research in patients at high risk of complications is now needed. Br Med J (Clinical Research edn) 333:1029–1030
Jungheinrich C, Scharpf R, Wargenau M, Bepperling F, Baron JF (2002) The pharmacokinetics and tolerability of an intravenous infusion of the new hydroxyethyl starch 130/0.4 (6 %, 500 mL) in mild-to-severe renal impairment. Anesth Analg 95:544–551
Ertmer C, Kampmeier TG, Rehberg S, Morelli A, Kohler G, Lange M, Bollen Pinto B, Hohn C, Hahnenkamp K, Van Aken H, Westphal M (2011) Effects of balanced crystalloid vs. 0.9 % saline-based vs. balanced 6 % tetrastarch infusion on renal function and tubular integrity in ovine endotoxemic shock*. Crit Care Med 39:783–792
Acknowledgments
The authors thank B. Kabisch, PhD, M. Muecke, and D. Schwarzkopf for their support in data analysis and J. Maltagliati for editing assistance. No compensation was received for these contributions. Funds for this project were provided by the Intramural Research Program of the U.S. National Institutes of Health. C. Natanson and J. Sun are U.S. government employees, and both did the work on this paper as part of their official U.S. government-funded research duties. However, the opinions expressed do not necessarily represent those of the U.S. National Institutes of Health.
Conflicts of interest
C. Hartog, H. Skupin, J. Sun, and C. Natanson declare that they have no conflict of interest. K. Reinhart has in the past received an unrestricted grant for the conduct of the VISEP study and speaker’s and consultancy fees from B. Braun, Melsungen, Germany. B.Braun, Melsungen also contributed to the German Sepsis Society to fund an endowed professorship for clinical sepsis research at the University Hospital of Jena.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Hartog and H. Skupin contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1
Electronic search strategy
Keywords: hetastarch OR hydroxyethyl starch OR hydroxyethylstarch OR pentastarch OR haes-steril OR Hextend OR Elohes OR Elohaes OR Expafusin OR Voluven OR hemohes OR hespan OR pentafraction OR pentaspan OR plasmasteril OR plasma expander OR hydroxyethylstarke OR hydroxyathylstarke OR hydroxyethyl starke OR HAES OR hydroxyethylamidon OR (colloid and crystalloid) AND fluid therapy OR fluid OR intensive care OR emergency OR burn OR organ transplantation OR donor resuscitation OR fluid resuscitation OR volume or hemodilution OR head injury OR stroke OR brain injury OR cerebral injury OR intracranial bleeding OR intravascular therapy OR plasma substitutes OR plasma expanders OR remplissage OR volumentherapy OR volumenersatz or plasmaersatz) AND review OR meta-analysis OR systematic review OR narrative review OR overview OR metaanalysis OR pooled analysis.
Appendix 2
The OQAQ is a validated instrument which has been used before in the setting of critical care [40] and anesthesia [41]. The OQAQ quality features according to Oxman et al. [27] and Delaney et al. [40] are as follows:
-
1
Were the search methods used to find evidence on the primary question(s) stated? (2) Was the search for evidence reasonably comprehensive?
-
2
Was the search for evidence reasonably comprehensive?
-
3
Were the criteria used for deciding which studies to include in the overview reported?
-
4
Was bias in the selection of studies avoided?
-
5
Were the criteria used for assessing the validity of the included studies reported?
-
6
Was the validity of all the studies referred to in the text assessed using appropriate criteria?
-
7
Were the methods used to combine the findings of the relevant studies (to reach a conclusion) reported?
-
8
Were the findings of the relevant studies combined appropriately relative to the primary question of the overview?
-
9
Were the conclusions made by the author(s) supported by the data and/or analysis reported in the overview?
-
10
How would you rate the overall quality of this overview?
Possible answers to questions 1–9 are: 1 = no; 2 = partially or cannot tell; 3 = yes. Answer to question 10 is: if the methods that were used are reported incompletely relative to a specific item, that item should be scored as “partially.” Similarly, if there is no information provided regarding what was done relative to a particular question, this question should be scored as “cannot tell,” unless there is information in the overview to suggest either that the criterion was or was not met.
For question 2, for a search to be considered comprehensive the methods used to perform the search should include searching for unpublished material as well as multiple medical databases (EMBASE as well as MEDLINE). If only published material was searched for, the search should be assessed as “partially” For question 4, for bias to have been avoided in the selection of studies, the report should indicate that explicit criteria were used to define studies eligible for inclusion. For question 6, to determine whether the validity was assessed using appropriate criteria, all of the studies in the text must have had their validity assessed and explicit criteria which were appropriate for the type of research question that was being addressed must have been used. For question 8, if no attempt has been made to combine findings, and no statement is made regarding the inappropriateness of combining findings, check “no.” If a summary (general) estimate is given anywhere in the abstract, the discussion, or the summary section of the paper, and it is not reported how that estimate was derived, check off “no,” even if there is a statement regarding the limitations of combining the findings of the studies reviewed. If in doubt, check off “can’t tell.” For an overview to be scored as “yes” on question 9, data (not just citations) must be reported that support the main conclusions regarding the primary question(s) that the overview addresses. The score for question 10, the overall scientific quality, should be based on your answers to the first nine questions. The following guidelines can be used to assist with deriving a summary score: if the “cannot tell” option is used one or more times on preceding questions, a review is likely to have minor flaws at best, and it is difficult to rule out major flows (i.e., a score ≤4). If the “no” option is used on questions 2, 4, 6, or 8, the review is likely to have major flaws (i.e., a score of ≤3, depending on the number and degree of the flaws).
Rights and permissions
About this article
Cite this article
Hartog, C.S., Skupin, H., Natanson, C. et al. Systematic analysis of hydroxyethyl starch (HES) reviews: proliferation of low-quality reviews overwhelms the results of well-performed meta-analyses. Intensive Care Med 38, 1258–1271 (2012). https://doi.org/10.1007/s00134-012-2614-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2614-0