Abstract
Aims/hypothesis
Several publications suggest an association between certain types of insulin and cancer, but with conflicting results. We investigated whether insulin glargine (A21Gly,B31Arg,B32Arg human insulin) is associated with an increased risk of cancer in a large population-based cohort study.
Methods
Data for this study were obtained from dispensing records from community pharmacies individually linked to hospital discharge records from 2.5 million individuals in the Netherlands. In a cohort of incident users of insulin, the association between insulin glargine and other insulin analogues, respectively, and cancer was analysed in comparison with human insulin using Cox proportional hazard models with cumulative duration of drug use as a time-varying determinant. The first hospital admission with a primary diagnosis of cancer was considered as the main outcome; secondary analyses were performed with specific cancers as outcomes.
Results
Of the 19,337 incident insulin users enrolled, 878 developed cancer. Use of insulin glargine was associated with a lower risk of malignancies in general in comparison with human insulin (HR 0.75, 95% CI 0.71, 0.80). In contrast, an increased risk was found for breast cancer (HR 1.58, 95% CI 1.22, 2.05). Dose–response relationships could not be identified.
Conclusion/interpretation
Users of insulin glargine and users of other insulin analogues had a lower risk of cancer in general than those using human insulin. Both associations might be a consequence of residual confounding, lack of adherence or competing risk. However, as in previous studies, we demonstrated an increased risk of breast cancer in users of insulin glargine in comparison with users of human insulin.
Similar content being viewed by others
Introduction
Diabetes mellitus is an important risk factor for cardiovascular disease [1, 2]. In addition, diabetes has been associated with an increased risk of colorectal cancer [3, 4], breast cancer [4, 5], endometrial cancer [4, 6], hepatocellular carcinoma [4, 7], pancreatic cancer [4, 8] and bladder cancer [4, 9]. In contrast, patients with diabetes have a decreased risk of developing prostate cancer [4, 10]. Furthermore, diabetes has been reported as an independent predictor of mortality from cancer [4, 11, 12]. However, due to factors such as duration of diabetes, different drugs used to attain metabolic control and presence of other diseases, the assessment of cancer risk in diabetes patients remains difficult [13, 14].
In 2004, a publication with data from the General Practice Research Database in the UK reported that in patients with type 2 diabetes, chronic insulin therapy was associated with a significantly higher risk of colorectal cancer compared with patients with diabetes who did not use insulin [15]. By the end of 2009, articles were published using data from population registries to analyse a possible relationship between the use of hypoglycaemic agents and the risk of cancer [16–19]. Of these, three showed an increased risk of cancer with use of insulin glargine (A21Gly,B31Arg,B32Arg human insulin) compared with other types of insulin analogues or human insulin [16, 18, 19]. Currie et al. did show an increased risk of cancer while using insulin compared with patients using metformin but did not show an increased risk of cancer for those using insulin analogues compared with those using human insulin [17]. More recently, it has been reported that the use of insulin glargine did not increase the risk of overall cancer compared with the use of human insulin [20].
In addition to these observational studies, reports regarding randomised controlled trials have been published [21–23]. None of these described dissimilarity in cancer incidence between participants treated with insulin glargine and those treated with human insulin or other types of insulin [21–23]. With regard to dose, a dose-dependent relationship has been described for insulin glargine and risk of cancer, but not for other insulin analogues or human insulin [18, 24]. Consequently, whether different types of insulin may be a cause of cancer is an issue of ongoing debate [25–32].
Therefore, the objective of this study was to analyse the hypothesis that use of insulin glargine is associated with an increased risk of cancer in comparison with use of human insulin.
Methods
Setting
Data for this study were obtained from the PHARMO Record Linkage System (PHARMO RLS), which includes drug-dispensing records from community pharmacies linked on a patient level to hospital discharge records from the Dutch National Medical Register for approximately 2.5 million individuals in the Netherlands since 1986 [33, 34].
The drug-dispensing database contains the following information per prescription as of 1998: anatomical therapeutic chemical (ATC) classification of the drug, dispensing date, regimen, quantity dispensed and estimated duration of use [35]. The hospital record database contains detailed information concerning primary and secondary discharge diagnoses and dates of admission and discharge. All diagnoses are coded according to the International Classification of Disease, ninth edition (ICD-9) [36].
Study population
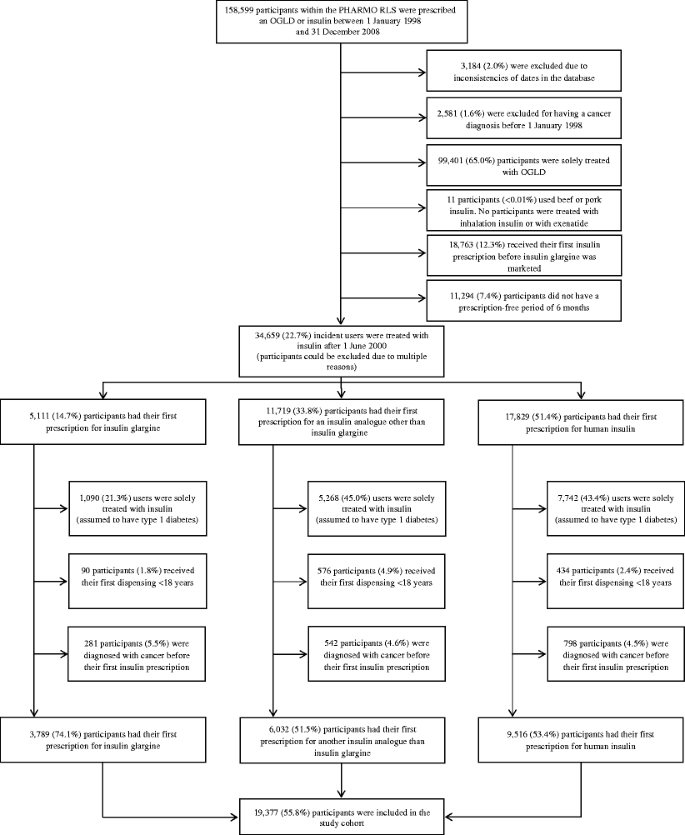
All participants with a prescription for any hypoglycaemic agent, including an oral glucose-lowering drug (OGLD) or insulin, between 1 January 1998 and 31 December 2008 were included in the study cohort. The patient flow is presented visually in a flow diagram (Fig. 1). As insulin glargine has been marketed in the Netherlands since June 2000, participants with a prescription of any insulin before 1 June 2000 were excluded from the cohort [37]. Furthermore, to ensure the study cohort included only incident insulin users, users needed to have had a 6 month period without prescription of insulin (any type) before inclusion. To mimic a study cohort of participants with type 2 diabetes, those using only insulin were assumed to have type 1 diabetes and were excluded from the analysis. In addition, participants with a primary cancer diagnosis before 1 June 2000, a primary cancer diagnosis before prescription of insulin, or who were aged under 18 years at first prescription were excluded. As a consequence, the remaining cohort only included insulin users with prior use of OGLD who were followed over time starting from the first prescription for insulin.
Exposure
The different types of insulin prescribed for diabetes were classified into three mutually exclusive categories according to ATC code: insulin glargine; other insulin analogues; and human insulin (electronic supplementary material [ESM] Table 1). For each participant, the number of cumulative days of insulin use was calculated. The cumulative exposure to each insulin category at any point in time during follow-up was calculated for each participant in days since start of the respective insulin type. Cumulative days of insulin exposure were taken from this time point until death of the participant, end of study, first diagnosis of cancer, relocation out of the PHARMO RLS catchment area, or the last day of use of a dispensed agent in the same insulin category.
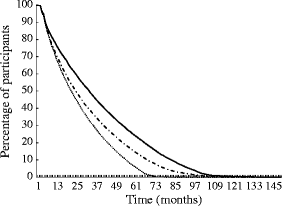
To visualise participants’ drug adherence with different types of insulin, the percentage of participants adherent to therapy was calculated. For every cohort member, the follow-up time was calculated for insulin glargine, other insulin analogues and human insulin, respectively. For every month of follow-up, the number of users was divided by the total number of those who started minus those who died, those diagnosed with cancer and those who moved out of the PHARMO RLS catchment area.
Outcome
The first hospital admission with a primary diagnosis of any type of cancer, ICD-9 codes 140–172, 174–209 and 235–239, was considered the primary outcome [36]. The secondary outcome measure was diagnosis of one of the following solid cancers: colon cancer (ICD-9 153 or 154), pancreatic cancer (ICD-9 157), breast cancer (ICD-9 174 or 175), prostate cancer (ICD-9 185), endometrial cancer (ICD-9 179 or 182), respiratory tract cancer (ICD-9 160–165) and bladder cancer (ICD-9 188). These cancers were selected because they have been associated with diabetes, either with an increased or with a decreased risk [3, 5, 6, 8–10].
Covariables
Age at first insulin prescription, sex, number of unique other drugs used in the year before start of insulin (excluding those prescribed for diabetes), number of hospitalisations in the year before start of insulin and calendar time were considered potential confounders or effect modifiers. The number of days of use of OGLD in the year before start of insulin therapy was calculated, as well as the number of OGLDs used as of 1 January 1998 to adjust for duration of diabetes. Furthermore, the average dose was calculated per insulin category as average defined daily dose (DDD) over the previously dispensed prescriptions to adjust for severity of glucose intolerance. For all types of insulin, one DDD is equivalent to 40 U insulin [35].
Statistical analysis
Calculation of cumulative exposure Individuals were followed from their first insulin prescription until the first of one of the following events: a cancer as defined above, death, end of data collection in the PHARMO RLS (i.e. the patient moves out of the PHARMO RLS area) or end of the study period at 31 December 2008. The association between insulin and cancer was analysed using Cox proportional hazard models with duration of cumulative drug use as a time-varying determinant, as described by Stricker and Stijnen [38]. In this model, cumulative exposure in participants with cancer at the date of diagnosis is compared with cumulative exposure in all individuals without cancer with the same duration of insulin exposure in days. Time since start of insulin is used as the underlying timescale in the Cox proportional hazards model. We assumed that cancer risk after a certain cumulative exposure does not return to zero after stopping (i.e. in case of switching to another type of insulin). However, time since cessation was taken into account in one of the sub-analyses. In the analysis performed, the actual exposure during follow-up was used. This analysis defines the exposure accurately but may suffer from reverse causation bias. To address this issue, analyses were performed taking into account a latent period before the diagnosis of cancer in which we assumed that cancer was already present 1 year before it was actually diagnosed (for instance, cumulative exposure to 21 June 2007 instead of 21 June 2008). To further deal with the issue of reverse causation, a fixed-cohort analysis was performed in which the first exposure to insulin determined the drug category in which the participant was categorised. To further address potential residual confounding, a propensity-score analysis was performed. The methods and results for the fixed and propensity-score analyses are presented in, respectively, ESM methods and results.
The ways in which use of OGLD and dose were addressed in the analyses are described in ESM methods, as are the general statistical methods used.
Results
Setting and characteristics
Within the PHARMO RLS, 158,599 participants were prescribed an OGLD or insulin between 1 January 1998 and 31 December 2008. After applying exclusion criteria, 19,337 (12.2%) participants were included in the study cohort (Fig. 1).
As can be seen from Table 1, there were significant differences at baseline and during follow-up between participants starting on insulin glargine or other insulin analogues and those starting on human insulin. Users of insulin analogues were significantly younger than those starting on insulin glargine; in contrast, those starting on insulin glargine were more frequently male than those starting on other insulin analogues. The mean number of unique other drugs used and number of hospitalisations in the year before start of insulin did not differ significantly. The first dose prescribed, as well as the average dose calculated over all prescriptions, was significantly lower for those using other insulin analogues in comparison with those using insulin glargine. The duration of OGLD use prior to start of insulin was significantly shorter for those using other insulin analogues than for those using insulin glargine or human insulin. However, when stratifying for the year in which insulin therapy was started, no clear differences could be seen (ESM Table 2). Last, the duration of days of follow-up since the start of insulin was considerably lower for users of insulin glargine than for those using other insulin analogues. An adherence curve is presented in Fig. 2 in which the percentages of participants adherent to the three different categories of insulin are visualised. Those dispensed insulin glargine were statistically significantly less adherent to therapy than those dispensed other insulin analogues or human insulin. In ESM Fig. 1 (insulin glargine), ESM Fig. 2 (other insulin analogues) and ESM Fig. 3 (human insulin) adherence is presented separately for those who died, those who got diagnosed with cancer and those who were censored at the end of study.
As-treated analyses
Of the 878 participants hospitalised for cancer, 158 were treated with insulin glargine, 423 with other insulin analogues and 592 participants were treated with human insulin. The corresponding incidence rates were, respectively, 11.29, 13.78 and 12.81 cancers per 1,000 patient years. As can be seen from Table 2, use of insulin glargine was associated with a lower risk of malignancies in comparison with use of human insulin (HR 0.71, 95% CI 0.67, 0.75). In the full model, adjustments did not change the HR (HR 0.75, 95% CI 0.71, 0.80). Stratifying for prior OGLD use for less or longer than 1 year did not change this point estimate nor did adjustment for prior days of OGLD used change the point estimates by more than 10%. Adjustments were made by adding dose as an additional time-varying covariable to the model (HR 0.75, 95% CI 0.71, 0.80) but, as follow-up information was used when applying this method, results from analyses stratified for baseline dose are also presented in Table 2. As the majority of the cohort members had a median first dose of 16.7 U per day (Table 1) these analyses were stratified in three strata: more than, less than or equal to the median dose per day. When replacing cumulative exposure at the end of follow-up with attained cumulative exposure 1 year prior to the diagnosis of cancer (in order to minimise the chance of reverse causation) the point estimates remained statistically significantly protective. Proportionality of the full model was tested; p values for insulin glargine and other insulin analogues were, respectively, 0.14 and 0.32.
When specific cancers were used as endpoints (Table 3) applying the full model, insulin glargine was associated with a significantly lower risk of colon cancer but not of other cancers. In contrast, use of insulin glargine was associated with an increased risk of breast cancer (HR 1.58, 95% CI 1.22, 2.05) and prostate cancer (HR 2.76, 95% CI 1.32, 5.80) in comparison with use of human insulin. The complete analyses for endometrial cancer and pancreatic cancer were not possible because of the low number of cancer diagnoses. Furthermore, with regard to the stratified model for the first prescribed dose, analyses were not possible for some of the lowest strata because of the low number of cases (≈70% of the participants received a first dose of 16.7 U per day [Table 1]). No clear dose effect could be seen over the different strata of dose. For other insulin analogues, no increased risk of breast cancer or prostate cancer was seen; in addition, no decreased risk of colon cancer was found. However, a decreased risk of bladder cancer as well as respiratory tract cancer was seen (Table 3).
In users of insulin glargine the dose was not related to the diagnosis of cancer (crude HR comparing those with an average DDD higher than the median with those having an average DDD lower than the median 1.02, 95% CI 0.77, 1.34, HR applying full model 0.98, 95% CI 0.74, 1.29) nor could this be demonstrated for insulin analogues other than insulin glargine (crude HR 1.02, 95% CI 0.99, 1.04; HR applying full model 0.95, 95% CI 0.76, 1.18) or for human insulin (HR 0.95, 95% CI 0.82, 1.09, HR applying a comparable full model 0.96, 95% 0.82, 1.12).
Fixed-cohort analyses and propensity-score analyses
For cancer in general, similar estimates were found in the fixed analyses (ESM Table 3). Comparable estimates were also gained from the propensity-score analyses; these results are presented in the ESM results. In the analyses with specific cancers as endpoints, the results differed slightly. With regard to insulin glargine, the decreased risk of colon cancer and the increased risk of breast cancer were nearly similar; however, for prostate cancer, no risk deviations could be found. The results for lung cancer were comparable, but an increased risk was found for bladder cancer. With regard to other insulin analogues, the results were comparable: no increased risk of breast cancer or prostate cancer was seen and no decreased risk of colon cancer was found. However, a decreased risk of bladder cancer as well as respiratory tract cancer was seen (ESM Table 4). As in the as-treated analyses, no dose–response relationships could be determined.
Discussion
In this study, we found that cumulative use of insulin glargine was associated with a significantly lower risk of cancer in general, and of colon cancer specifically, in comparison with use of human insulin. Similar results were found for the risk of cancer in general and use of other insulin analogues in comparison with human insulin. In contrast, as in other studies, we found an increased risk of breast cancer for insulin glargine in comparison with human insulin [16, 19]. However, this has not been consistently confirmed by others [17, 22–24, 39, 40]. For insulin analogues other than insulin glargine, no increased risk of breast cancer was found. With regard to breast cancer, insulin glargine has shown a significantly higher proliferative effect on breast cancer cells compared with human insulin or other insulin analogues [41]. Recently, it was estimated that the serum of type 1 diabetic patients containing insulin glargine was 1.11-fold more mitogenic than human insulin containing serum [42]. Our results for other specific cancers as outcomes were not consistent, with the exception of a decreased risk of colon cancer for the use of insulin glargine and a decreased risk of bladder cancer and respiratory tract cancer for the use of other insulin analogues.
It might be hypothesised that the protective effect of insulin glargine on cancer in general is a result of the lower dose prescribed to these participants in comparison with the dose prescribed to participants using other insulin analogues or human insulin. Adjustment for dose was performed by adding dose as a time-dependent covariable in the model. Using this method, follow-up information is used, which is prone to reverse causality bias. Therefore, analyses were stratified for the baseline dose. However, in these stratified analyses as well as separate dose analyses, no dose-dependent relations could be demonstrated.
Our results are partly at variance with the earlier published population-based studies that caused alarm [16–20]. The first of these papers concluded that risk of cancer in participants using insulin glargine was higher than in those using human insulin [18]. As a possible explanation, the mitogenic properties of insulin glargine in diabetic patients, as published earlier, were suggested [43]. Another study reported that insulin analogues were not associated with a higher incidence of cancer compared with human insulin [17]. The third one, a Swedish study, did not show an increased risk of any malignancy, but similar to our study, they showed that women using insulin glargine had an increased incidence rate of breast cancer compared with women using other types of insulin analogues or human insulin [19]. The Scottish Diabetes Research Network found that those receiving insulin glargine had the same incidence rate for all cancers as those not receiving insulin glargine [16]. However, a subset of patients using insulin glargine alone had a significantly higher incidence of all cancers, and breast cancer specifically, than those using other types of insulin [16]. Nevertheless, the authors concluded that insulin glargine use was most likely not associated with an increased risk of cancer and that the finding above should be considered to be biased because of differences in allocation of patients to different types of insulin [16]. More recently, a cohort study of new users of OGLDs showed that the number of insulin doses dispensed (any insulin type) was associated with a higher risk of cancer compared with participants not using insulin [44]. In contrast, it was reported that, in a Taiwanese cohort study [20], use of insulin glargine was not associated with an increased risk of overall cancer while in Chinese individuals with type 2 diabetes, insulin usage (any type) was associated with a reduced risk of cancer compared with non-usage [45]. However, the latter study was severely criticised for the exclusion of follow-up time prior to insulin use [25].
Limitations of the earlier publications were brought forward, among which were short follow-up, failure to correct for body mass index, the impossibility of breaking down the risk of cancer in general to a tumour-specific risk, the inability to consider prior use of insulin before start of study, low numbers of patients using a specified insulin and the absence of dose analyses [32]. In addition, clinical decisions determining each patient’s treatment are not random and confounding by severity of glucose intolerance could play an important role in observational studies [28, 30]. Another issue is reverse causality and assessment of aetiologically relevant timing of exposure: cancer has a long latency period during which the disease itself may cause changes in treatment [28, 30]. Last, the severity of disease may also be related to the frequency of clinical contact, which may reduce the time between onset and diagnosis of cancer [28].
As described above, reverse causality may play a role in observational studies, as cancer often has a long latency period between the biological onset of the disease and the clinical diagnosis. During this latency period, symptoms related to still undetected cancer may lead to treatment changes. By cumulating exposure to 1 year prior to the diagnosis of cancer, we attempted to minimise reverse causality by taking into account a latent period (i.e. when the disease is already present but not yet diagnosed). To further address reverse causation, we performed a fixed analysis; none of these analyses changed the risk of cancer in general by more than 10%. To address the issue of potential residual confounding a propensity-score analysis was performed from which similar estimates were found. Also, although we assumed that cancer risk does not return to the background rate after a certain cumulative exposure, we performed a sensitivity analysis in which we adjusted for time since cessation. This adjustment was done to investigate whether the risk declined after discontinuation. However, this did not substantially change the risk estimates.
Our study was performed in incident users of insulin: those who had a prescription-free period of 6 months before study entry. By excluding those with prevalent use of insulin, we attempted to make participants more comparable with regard to duration and severity of insulin resistance. However, the participants being prescribed insulin glargine differed considerably from those being prescribed other insulin analogues or human insulin. Insulin glargine is reserved for those suffering from nightly hypoglycaemic attacks, partly because of its higher cost in comparison to human insulin [37]. Patients with type 1 diabetes are particularly prone to these attacks as, in contrast to patients with type 2 diabetes, they do not have any remaining insulin production [1]. However, it is possible that under everyday circumstances in the Netherlands, insulin glargine is prescribed more generally to those having difficulties attaining euglycaemia. Unfortunately, we were not able to fully differentiate between those receiving insulin for type 1 or for type 2 diabetes; these groups might differ regarding their cancer risk. However, in an attempt to restrict the analysis to those with type 2 diabetes, we included only participants with prior OGLD use. We were able to adjust for the number of unique other drugs used prior to the first prescription of insulin and the number of hospitalisations to adjust for comorbidity. Nevertheless, it is likely that our findings are confounded as those receiving insulin glargine or other insulin analogues might die earlier because of comorbidity; consequently they would not live long enough to develop cancer or, in other words, they would die of ‘competing risks’ [46]. Another explanation for our findings might be the significantly lower adherence to insulin glargine in comparison with use of other insulin analogues or human insulin.
In contrast to some former studies, we were not able to adjust for smoking status or body mass index, which might be considerable confounding factors. However, although obesity is associated with an increased risk of developing insulin resistance and type 2 diabetes [47] caution must be made when assessing the relationship with cancer. Furthermore, in previous studies, smoking and body mass index did not change the point estimate by more than 10% [16, 19].
Last, in our study we used cancer hospitalisation as an outcome measure, which is different from pathology data for cancer diagnoses. Some cancers might be diagnosed more frequently in a non-clinical setting. Within each specific cancer, this would, however, lead to non-differential misclassification of the outcome and consequently to dilution of the estimated effect towards the null hypothesis.
In conclusion, in our study of insulin users, users of insulin glargine had a lower risk of specific cancers and of cancer in general in comparison with those on human insulin. Similar results were found for use of other insulin analogues in comparison with human insulin. However, in our opinion, both associations might be a consequence of residual confounding, lack of adherence or competing risk. The fact that we were not able to demonstrate a dose–effect association would also be an argument against a causal relationship. Furthermore, as in previous studies, we demonstrated an increased risk for breast cancer and use of insulin glargine [16, 19]. In our opinion, reasons for concern with regard to the safety of insulin glargine remain and the possible association with cancer, and breast cancer specifically, requires further attention.
Abbreviations
- ATC:
-
Anatomical therapeutic chemical
- DDD:
-
Defined daily dose
- OGLD:
-
Oral glucose-lowering drug
- PHARMO RLS:
-
PHARMO Record Linkage System
References
Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J (2008) Harrison's principles of internal medicine, 17th edition. McGraw-Hill, New York
Collaboration Emerging Risk Factors, Sarwar N, Gao P et al (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222
Larsson SC, Orsini N, Wolk A (2005) Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 97:1679–1687
Giovannucci E, Harlan DM, Archer MC et al (2010) Diabetes and cancer: a consensus report. Diabetes Care 33:1674–1685
Larsson SC, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 121:856–862
Friberg E, Orsini N, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 50:1365–1374
Hassan MM, Curley SA, Li D et al (2010) Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 116:1938–1946
Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M (2005) Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 92:2076–2083
Larsson SC, Orsini N, Brismar K, Wolk A (2006) Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 49:2819–2823
Bonovas S, Filioussi K, Tsantes A (2004) Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia 47:1071–1078
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 159:1160–1167
van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR (2007) Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer 120:1986–1992
Grote VA, Becker S, Kaaks R (2010) Diabetes mellitus type 2—an independent risk factor for cancer? Exp Clin Endocrinol Diabetes 118:4–8
Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R (2009) Diabetes and cancer. Endocr Relat Cancer 16:1103–1123
Yang YX, Hennessy S, Lewis JD (2004) Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology 127:1044–1050
Colhoun HM, SDRN Epidemiology Group (2009) Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 52:1755–1765, Erratum 52: 2469
Currie CJ, Poole CD, Gale EA (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52:1766–1777
Hemkens LG, Grouven U, Bender R et al (2009) Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 52:1732–1744
Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G (2009) Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia 52:1745–1754
Chang CH, Toh S, Lin JW et al (2011) Cancer risk associated with insulin glargine among adult type 2 diabetes patients—a nationwide cohort study. PLoS One 6:e21368
Dejgaard A, Lynggaard H, Rastam J, Krogsgaard Thomsen M (2009) No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: a meta-analysis. Diabetologia 52:2507–2512
Home PD, Lagarenne P (2009) Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia 52:2499–2506
Rosenstock J, Fonseca V, McGill JB et al (2009) Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study. Diabetologia 52:1971–1973
Mannucci E, Monami M, Balzi D et al (2010) Doses of insulin and its analogues and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 33:1997–2003
Carstensen B (2010) Comment on: Yang et al. (2010) Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes; 59:1254–1260. Diabetes 59: e17–18; author reply e19–22
Edwards KL, Riche DM, Stroup JS et al (2010) Insulin glargine and cancer risk: an opinion statement of the endocrine and metabolism practice and research network of the American College of Clinical Pharmacy. Pharmacotherapy 30:955–965
Gerstein HC (2010) Does insulin therapy promote, reduce, or have a neutral effect on cancers? JAMA 303:446–447
Hernandez-Diaz S, Adami HO (2010) Diabetes therapy and cancer risk: causal effects and other plausible explanations. Diabetologia 53:802–808
Johnson JA, Gale EA (2010) Diabetes, insulin use, and cancer risk: are observational studies part of the solution-or part of the problem? Diabetes 59:1129–1131
Pocock SJ, Smeeth L (2009) Insulin glargine and malignancy: an unwarranted alarm. Lancet 374:511–513
Simon D (2010) Diabetes treatment with insulin glargine and risk of malignancy: methodological pitfalls and ethical issues. Diabetologia 53:204–205
Smith U, Gale EA (2009) Does diabetes therapy influence the risk of cancer? Diabetologia 52:1699–1708
Dutch Hospital Data, DBC Information System 1998–2008. Available from www.swov.nl/nl/research/kennisbank/inhoud/90_gegevensbronnen/inhoud/lmr.htm, accessed 1 January 2010
Herings R (1993) PHARMO: a record linkage system for postmarketing surveillance of prescription drugs in the Netherlands. Thesis, Department of Pharmacoepidemiology and Pharmacotherapy Utrecht, Utrecht University, 207
WHO Collaborating Centre for Drug Statistics Methodology—ATC/DDD Index. Available from www.whocc.no/atc_ddd_index, accessed 1 January 2010
WHO (1992) International Statistical Classification of Diseases and Related Health Problems, ninth edition. Available from www.icd9cm.chrisendres.com/, accessed 1January 2010
MEB The Dutch Medicines Evaluation Board - Database of Human Medicines. Available from www.cbg-meb.nl/CBG/nl/humane-geneesmiddelen/geneesmiddeleninformatiebank/default.htm, accessed 1 July 2010
Stricker BH, Stijnen T (2010) Analysis of individual drug use as a time-varying determinant of exposure in prospective population-based cohort studies. Eur J Epidemiol 25:245–251
Suissa S, Azoulay L, Dell'aniello S, Evans M, Vora J, Pollak M (2011) Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia 54:2254–2262
Morden N, Liu S, Smith J, Mackenzie T, Skinner J, Korc M (2011) Further exploration of the relationship between insulin glargine and incident cancer—a retrospective cohort study of older Medicare patients. Diabetes Care 34:1965–1971
Mayer D, Shukla A, Enzmann H (2008) Proliferative effects of insulin analogues on mammary epithelial cells. Arch Physiol Biochem 114:38–44
Mayer D, Chantelau E (2010) Treatment with insulin glargine (Lantus) increases the proliferative potency of the serum of patients with type-1 diabetes: a pilot study on MCF-7 breast cancer cells. Arch Physiol Biochem 116:73–78
Kurtzhals P, Schaffer L, Sorensen A et al (2000) Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 49:999–1005
Bowker SL, Yasui Y, Veugelers P, Johnson JA (2010) Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia 53:1631–1637
Yang X, Ko GT, So WY et al (2010) Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes 59:1254–1260
Rothman K (2002) Epidemiology, an introduction. Oxford University Press, Oxford
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Contribution statement
All authors were involved in the conception and design of the study. The data were analysed and interpreted by RR, LV and BS, who also drafted the article. All authors revised the article critically for important intellectual content. All authors gave their final approval of the current version to be published.
Duality of interest
M. van Herk-Sukel and R. M. C. Herings are employees of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for government and related healthcare authorities, and for pharmaceutical companies. However, this study is not financially supported by a pharmaceutical company. The first and last author work at the Dutch Inspectorate of Healthcare. S. Strauss works at the Dutch Medicines Evaluation Board. None of the authors has declared any other conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
PDF 66.7 kb
ESM Table 1
PDF 14 kb
ESM Table 2
PDF 38 kb
ESM Table 3
PDF 17.6 kb
ESM Table 4
PDF 39.8 kb
ESM Fig. 1
PDF 39 kb
ESM Fig. 2
PDF 42 kb
ESM Fig. 3
PDF 42 kb
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ruiter, R., Visser, L.E., van Herk-Sukel, M.P.P. et al. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia 55, 51–62 (2012). https://doi.org/10.1007/s00125-011-2312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2312-4