Summary
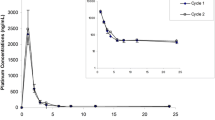
The pharmacokinetics of (glycolato-0,0′)-diammine platinum (II) (254-S; NSC 375101D), one of the new platinum analogues, was examined in a phase I study of this drug and compared with that of cisplatin and carboplatin. All drugs were given in short-term (30-min) i.v. drip infusions; the doses of 254-S, cisplatin, and carboplatin were 100, 80, and 450 mg/m2, respectively. Platinum concentrations in whole plasma, plasma ultrafiltrate, and urine were determined by atomic absorption spectrometry. After the infusion, the plasma concentration of total platinum for the three agents decayed biphasically. Ultrafilterable platinum in plasma decreased in a biexponential mode after infusions of 254-S and carboplatin, whereas the free platinum of cisplatin showed a monoexponential disappearance. The peak plasma concentrations and AUC for free platinum were 5.31 μg/ml and 959 μg/min per ml for 254-S, 3.09 μg/ml and 208 μg/min per ml for cisplatin, and 19.90 μg/ml and 3446 μg/min per ml for carboplatin, respectively. The mean ratio of plasma ultrafilterable platinum to total platinum were calculated, and the results showed that the protein-binding abilities of 254-S and carboplatin were almost identical. More than 50% of the 254-S was excreted in the urine within the first 480 min after its administration. Thrombocytopenia was reported as a dose-limiting toxicity for both 254-S and carboplatin. This similarity in side effects may mainly be due to the comparable pharmacokinetic behavior of these two platinum compounds.
Similar content being viewed by others
References
Einhorn LH (1981) Testicular cancer as a model of a curable neoplasma. The Richard and Hinda Rothenthal Foundation Award Lecture. Cancer Res 41:3275
Einhorn LH, Donohue J (1977) cis-Diamminedichloroplatinum, vinblastine and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med 87:293
Gralla RJ (1981) Cisplatin and vindesine combination chemotherapy for advanced carcinoma of the lung: a randomized trial investigating two dosage schedules. Ann Intern Med 95:414
Harrap KR, Jones M, Wilkinson CR, Clink HMcD, Sparrow S, Mitchley BCV, Clarke SA, Veasy A (1980) Antitumor, toxic and biochemical properties of cisplatin and eight other platinum complexes. In: Prestayko AW, Cooke ST, Carter SK (eds) Cisplatin. Current status and new developments. Academic Press, New York, p 193
Higby DJ, Wallance HJ Jr, Albert DJ, Holland JF (1977) Diamminedichloroplatinum: a phase I study showing response in testicular and other tumors. Cancer 33:1219
Kon S, Sarcozi L (1976) Platinum determination in blood and biological tissues by flameless atomic absorption. Clin Chem 22:1211
Leyvraz S, Ohnuma T, Lassus M, Holland F (1985) Phase I study of carboplatin in patients with advanced cancer, intermittent intravenous bolus, and 24-hour infusion. J Clin Oncol 3:1385
Rosenberg B, Van Camp L, Krigas T (1965) Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205:698
Rosenberg B, Van Camp L, Trasko JE, Mansour VH (1969) Platinum compounds: a new class of potent anti-tumor agents. Nature 222:385
Sasaki Y, Saijo N, Tamula T, Eguchi K, Shinkai T, Nakano H, Nakagawa K (1987) Comparison of the antitumor activity of cisplatin and its derivatives with special stress on the pharmacokinetics of active form of drugs in the plasma determined by colony assay. Proc Am Soc Clin Oncol 6:34
Shinkai T, Saijo N, Tominaga K, Eguchi K, Shimizu E, Sasaki Y, Fujita J, Futami H (1985) Comparison of vindesine plus cisplatin or mitomycin C in the treatment of advanced non small cell lung cancer. Cancer Treat Rep 69:1945
Shiratori O, Kanai H, Uchida N, Takeda Y, Totani T, Sato K (1985) Antitumor activity of 254-S, a platinum complex, in rodents. In: Ishigami J (ed) Recent advances in chemotherapy. University of Tokyo Press, Tokyo, p 635
Soloway MS (1978) cis-Diamminedichloroplatinum(II) (DDP) in advanced bladder cancer. J Urol 120:716
Taguchi J, Saijo N, Miura K, Shinkai T, Eguchi K, Sasaki Y, Tamura T, Sakurai M, Minato K, Fujiwara Y, Nakano H, Nakagawa K, Hong WS (1987) Prediction of hematologic toxicity of carboplatin by creatinine clearance rate. Jpn J Cancer Res (Gann) 78:977
Takahashi K, Seki T, Nishikawa K, Minamide S, Horinishi H (1985) Antitumor activity and toxicity of serum proteinbound platinum formed from cisplatin. Jpn J Cancer Res (Gann) 76:68
Van Echo DA, Egorin MJ, Whitacre Y, Olman EA, Aisner J (1984) Phase I clinical and pharmacologic trial of carboplatin daily for 5 days. Cancer Treat Rep 68:1103
Wilkinson R, Cox PJ, Jones M, Harrap KR (1978) Selection of potential second generation platinum compounds. Biochemie 60:851
Williams CJ (1979) Advanced ovarian carcinoma: a pilot study of cis-dichlorodiammineplatinum(II) in combination with adriamycin and cyclophosphamide in previously untreated patients as a single agent. Cancer Treat Rep 63:1745
Wiltshaw E (1979) Cancer of the ovary: a summary of experience with cis-dichloroplatinum(II) at the Royal Marsden Hospita. Cancer Treat Rep 63:1545
Yogoda A (1977) Future implications of phase 2 chemotherapy trials in 96 patients with measurable advanced bladder cancer. Cancer Res 37:2275
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sasaki, Y., Tamura, T., Eguchi, K. et al. Pharmacokinetics of (glycolato-0,0′)-diammine platinum (II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother. Pharmacol. 23, 243–246 (1989). https://doi.org/10.1007/BF00451649
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00451649